
Concept explainers
(a)
Interpretation:
The principal organic product that is obtained when
Concept introduction:
The reaction in which an atom of
Answer to Problem 26.26AP
The principal organic product that is obtained when
Explanation of Solution
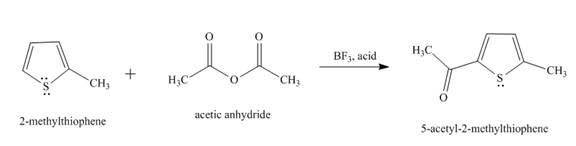
The reaction of
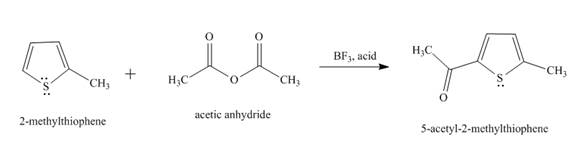
Figure 1
In the above reaction, the second position of
Therefore, the product formed by the above reaction is
The principal organic product,
(b)
Interpretation:
The principal organic product that is obtained when
Concept introduction:
The reaction in which an atom of aromatic system is replaced by an electrophile is known as electrophilic aromatic substitution reaction. The compound, acetic anhydride behaves as a acetylating compound in the presence of
Answer to Problem 26.26AP
The principal organic product that is obtained when
Explanation of Solution
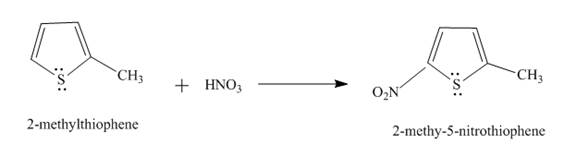
The reaction of
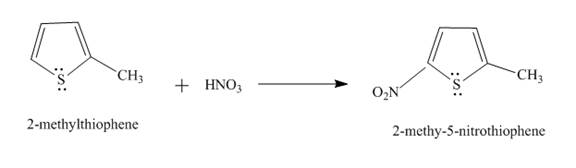
Figure 2
In the above reaction, the second position of
Therefore, the product formed by the above reaction is
The principal organic product,
(c)
Interpretation:
The incomplete reaction between diene and dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
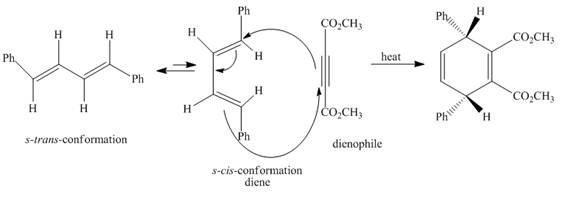
Explanation of Solution
The given incomplete reaction is shown below.
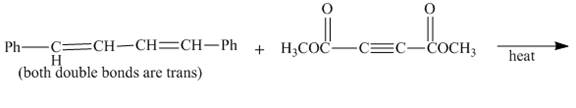
Figure 3
In the above incomplete reaction, the given
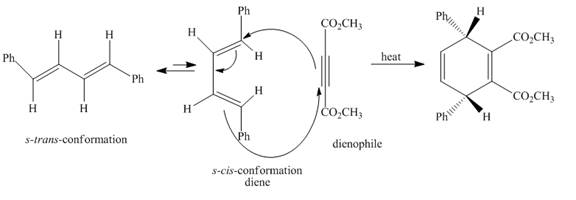
Figure 4
Therefore, one product is obtained from the above shown Diels Alder reaction.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 4.
(d)
Interpretation:
The incomplete reaction between an alkene and benzoquinone is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
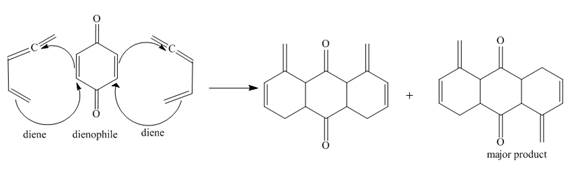
Explanation of Solution
The given incomplete reaction is shown below.
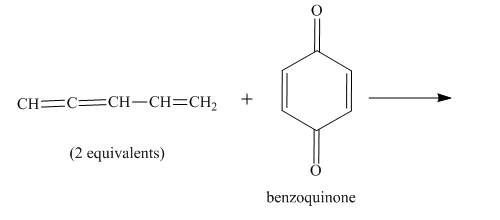
Figure 5
In the above incomplete reaction, the given two equivalents of alkene behaves as a diene and undergoes Diels Alder reaction with benzoquinone which behaves as a dienophile in the presence of heat to form two products as shown below.

Figure 6
Therefore, two products are obtained from the above shown Diels Alder reaction. The second product is the major one because of the less van der Waals repulsion present in between two double bonds which are exocyclic.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 6.
(e)
Interpretation:
The incomplete reaction between a diene and a dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
Explanation of Solution
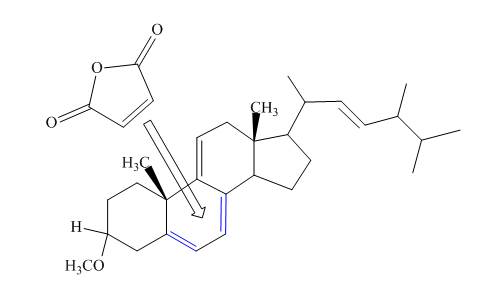
The given incomplete reaction is shown below.
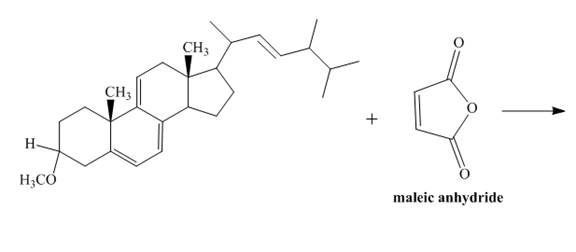
Figure 7
Only the

Figure 8
The above reaction does not form any product because the
Therefore, no product is formed in the above shown reaction.
There is no formation of the product takes place in the given reaction.
(f)
Interpretation:
The incomplete reaction between a diene and a dienophile is to be completed with the reasonable products. The reason corresponding to the formation of the correct product is to be explained.
Concept introduction:
Diels Alder reaction is the
Answer to Problem 26.26AP
The complete reaction is shown below.
Explanation of Solution
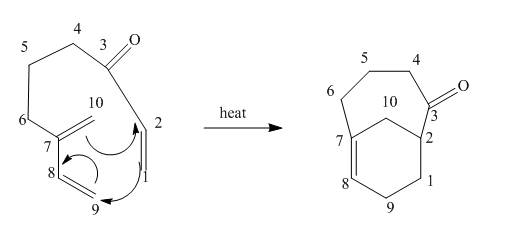
The given incomplete reaction is shown below.
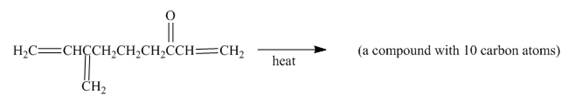
Figure 9
In the above incomplete reaction, the intramolecular Diels Alder reaction takes place in the presence of heat to form two products as shown below.

Figure 10
In the above reaction, diene and dienophile are present in the same compound. Therefore, two products are obtained from the above shown Diels Alder reaction. Therefore, the shifting of bonds takes place within the molecule to form a single product.
The complete reaction corresponding to the incomplete reaction between diene and dienophile is shown in Figure 10.
(g)
Interpretation:
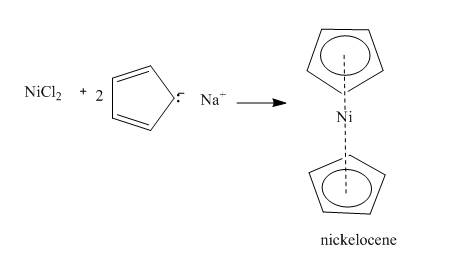
The incomplete reaction between nickel choride and
Concept introduction:
Metallocene compounds are composed of an electropositive metal ions specially
Answer to Problem 26.26AP
The complete reaction is shown below.
Explanation of Solution
The given incomplete reaction is shown below.
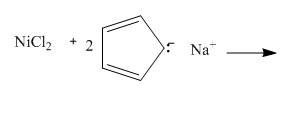
Figure 11
In the above incomplete reaction, the nickel chloride reacts with

Figure 12
Therefore, the reaction between nickel chloride and
The complete reaction corresponding to the incomplete reaction between nickel choride and
Want to see more full solutions like this?
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- The base-promoted rearrangement of an -haloketone to a carboxylic acid, known as the Favorskii rearrangement, is illustrated by the conversion of 2-chlorocyclohexanone to cyclopentanecarboxylic acid. It is proposed that NaOH first converts the a-haloketone to the substituted cyclopropanone shown in brackets and then to the sodium salt of cyclopentanecarboxylic acid. (a) Propose a mechanism for base-promoted conversion of 2-chlorocyclohexanone to the proposed intermediate. (b) Propose a mechanism for base-promoted conversion of the proposed intermediate to sodium cyclopentanecarboxylate.arrow_forward(a) Arrange the following compounds in an increasing order of their indicated property :(i) Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4-Methoxybenzoic acid (acid strength)(ii) CH3CH2CH (Br) COOH, CH3CH (Br) CH2COOH,(CH3)2CHCOOH, CH3CH2CH2COOH (acid strength)(b) How would you bring about the following conversions :(i) Propanone to Propene (ii) Benzoic acid to Benzaldehyde(iii) Bromobenzene to 1-phenylethanolarrow_forwardTo synthesize trans-cinnamic acid from benzaldehyde (PhCHO) and acetic anhydride (Ac2O) under basic, refluxing conditions to exemplify the Perkin condensation. 1. Draw the complete chemical reaction to be carried out. 2. Provide a complete arrow-pushing mechanism for the synthesis of trans-cinnamic acid. 3. Briefly describe the role of water, Na2CO3, and HCl in the isolation of the product. Draw the structure of the product at basic pH (after Na2CO3 addition) and at acidic pH (after HCl addition).arrow_forward
- (a) Write the structures of main products when aniline reacts with the following reagents :(i) Br2 water (ii) HCI (iii) (CH3CO)2O/pyridine(b) Arrange the following in the increasing order of their boiling point :C2H5NH2, C2H5OH, (CH3)3N(c) Give a simple chemical test to distinguish between the following pair of compounds : (CH3)2NH and (CH3)3Narrow_forwarda) Give the appropriate base for the above reaction and draw the resonance structures of the enolate ions derived from compound A. Then write the mechanism for the formation of the Dieckmann cyclized product B. b) If compound B above is reacted with NaBH 4 , draw the structure of the reduced product. Give a reason for your choice of product. c) Being a β-ketoester, B could undergo a 3-step synthesis involving alkylation, hydrolysis and decarboxylation reactions to yield the cyclopentanone, C. Write the outline synthesis for each step which include the appropriate regents and correct intermediate.arrow_forwardA. In the synthesis of 1-bromobutane, what is the inorganic by-product left in the reaction flask following the distillation? Why was the bromoalkane the bottom layer in the separatory funnel? B. Predict the product when 1-methylcyclohexanol reacts with H2SO4 and KBr. Show the mechanism.arrow_forward
- z When (R)-2-butanol is left standing in aqueous acid, it slowly loses its optical activity. Account for this observationarrow_forwardElectrophilic nitration of benzoic acid gives almost exclusively 3-nitrobenzoic acid. By drawing the appropriate resonance forms of the intermediate cations resulting from attack of [NO2]+, explain this result.arrow_forwarda. Compound X is benzene, Y is acetic anhydride acid. Complete the following scheme and determine Z! b. Determine which reagents except acetic acid anhydrides can replace Y!arrow_forward
- The 1H NMR spectra for two esters with molecular formula C8H8O2 are shown next. Which of the esters is hydrolyzed more rapidly in an aqueoussolution with a pH of 10?arrow_forwardAnilinium ion, PhNH3+ , Catalyze semicarbazone formation from benzaldhyde much more effectively than would be expected on the basis of its strength as an acid. Explain.arrow_forwardFollowing is a synthesis for toremifene, a nonsteroidal estrogen antagonist whose structure is closely related to that of tamoxifen. (a) This synthesis makes use of two blocking groups, the benzyl (Bn) group and the tetrahydropyranyl (THP) group. Draw a structural formula of each group and describe the experimental conditions under which it is attached and removed. (b) Discuss the chemical logic behind the use of each blocking group in this synthesis. (c) Propose a mechanism for the conversion of D to E. (d) Propose a mechanism for the conversion of F to toremifene. (e) Is toremifene chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
