
Concept explainers
(a)
Interpretation:
The reaction between
Concept introduction:
Deuterium is an isotope of hydrogen. Deuterium contains one neutron and one proton in its nucleus while hydrogen has only one proton in its nucleus but the number of electrons in hydrogen and deuterium is one.
Isotopic substitution is a reaction in which one isotope is substituted by the other
Answer to Problem 26.34AP
The complete reaction between
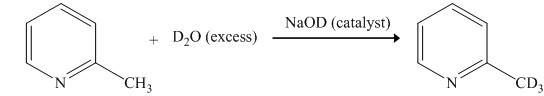
Explanation of Solution
The reaction to be completed is shown below.
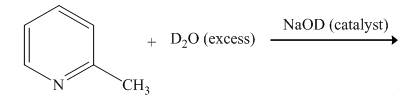
Figure 1
When

Figure 2
The complete reaction between
(b)
Interpretation:
The reaction between
Concept introduction:
Phenyllithium is an organometallic agent which is used to introduce metal into an organic compound during synthesis. It is used to give nucleophilic addition and substitution reactions.
Answer to Problem 26.34AP
The complete reaction between
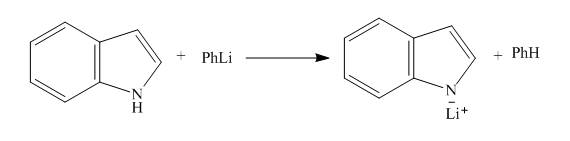
Explanation of Solution
The reaction to be completed is shown below.
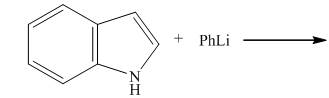
Figure 3
When indole reacts with phenyllithium, phenyllithium abstracts a proton bonded to nitrogen atom and a lithium salt of indole is formed as a conjugate base. The complete reaction is shown below.

Figure 4
The complete reaction between
(c)
Interpretation:
The reaction of
Concept introduction:
Electrophilic aromatic substitution reaction involves the substitution of an electrophile on an aromatic ring. The
Answer to Problem 26.34AP
The complete reaction of
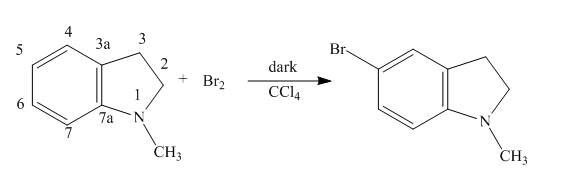
Explanation of Solution
The reaction to be completed is shown below.
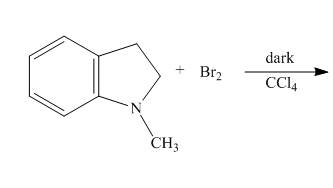
Figure 5
When
The complete reaction is shown below.

Figure 6
The complete reaction of
(d)
Interpretation:
The reaction of pyrrolizine with hydrogen in presence of platinum and carbon catalyst is to be completed by giving the major product.
Concept introduction:
Catalytic hydrogenation is a reduction process of addition of hydrogen atoms in an
Answer to Problem 26.34AP
The complete reaction of pyrrolizine with hydrogen in presence of platinum and carbon catalyst is shown below.
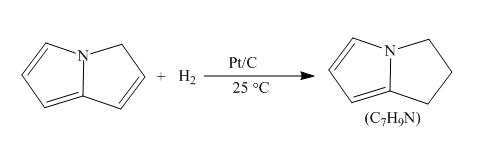
Explanation of Solution
The reaction to be completed is shown below.
c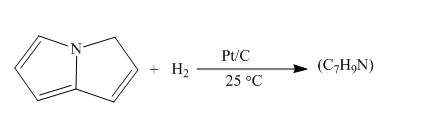
Figure 7
When pyrrolizine reacts with hydrogen in presence of

Figure 8
The complete reaction of pyrrolizine with hydrogen in presence of platinum and carbon catalyst is shown in Figure 8.
(e)
Interpretation:
The reaction of
Concept introduction:
Nitration is a process in which an
Answer to Problem 26.34AP
The complete reaction of
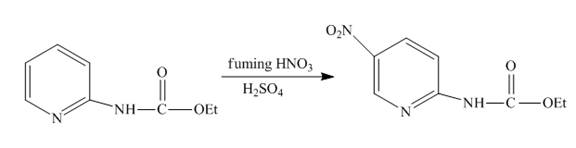
Explanation of Solution
The reaction to be completed is shown below.
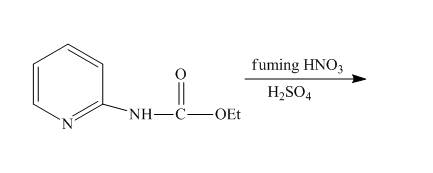
Figure 9
Nitration occurs when

Figure 10
The complete reaction of
(f)
Interpretation:
The reaction between
Concept introduction:
Electrophilic aromatic substitution reaction involves the substitution of an electrophile on an aromatic ring. The
Answer to Problem 26.34AP
The complete reaction between
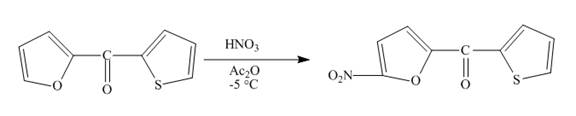
Explanation of Solution
The reaction to be completed is shown below.
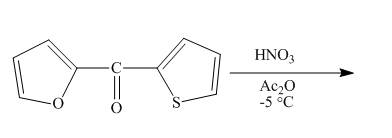
Figure 11
Electrophilic aromatic substitution reaction will occur in Furan since it is more reactive than thiophene. Therefore, nitration will occur at carbon

Figure 12
The complete reaction between
(g)
Interpretation:
The reaction of
Concept introduction:
Nitration is a process in which an aromatic compound is nitrated by electrophilic substitution mechanism in presence of concentrated sulfuric acid and concentrated nitric acid. Electron donating groups substituted on the aromatic ring are those which donates electron to the aromatic ring. Electron donating groups are ortho and para-directing.
Answer to Problem 26.34AP
The complete reaction of
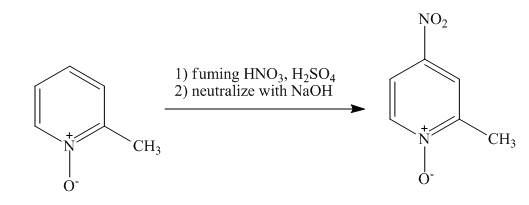
Explanation of Solution
The reaction to be completed is shown below.
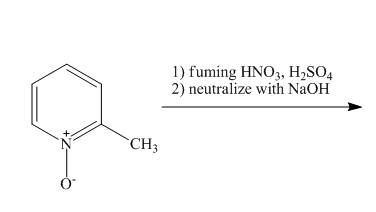
Figure 13
The pyridine ring activates towards electrophilic aromatic substitution due to the presence of pyridinium

Figure 14
The complete reaction of
(h)
Interpretation:
The reaction of
Concept introduction:
Wolff Kishner reduction is a reaction in which
Answer to Problem 26.34AP
The complete reaction of
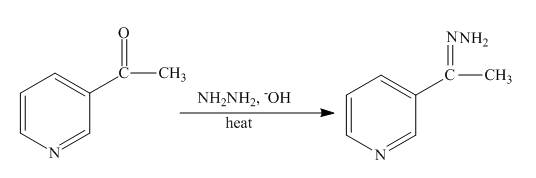
Explanation of Solution
The reaction to be completed is shown below.
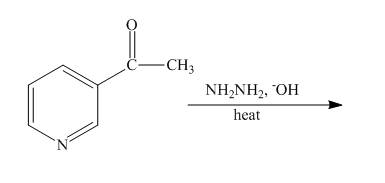
Figure 15
The Wolff-Kishner reduction takes place when

Figure 16
The complete reaction of
Want to see more full solutions like this?
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- When the nitrogen-containing aromatic heterocyclic compounds 1 and 2 are treated with HCl, only 1 forms the hydrochloride salt, whereas compound 2 is unreactive. Provide an explanation for this observed reactivity.arrow_forwardWrite the structure of the Maleic Anhydride compound and label each non-equivalent carbon with a letter, A,B,C...arrow_forwardCompound A is an alcohol that undergoes oxidation to produce compound B.Compound B is a ketone that gives positive triiodomethane reaction. Compound B isthen reacted with phenyl magnesium bromide, C6H5MgBr in the presence of aqueousacid to form compound C. Compound C has the molecular formula of C9H12O. Deducethe structure for compound A, B and C. PLEASE PROVIDE CLEAR DRAWINGS AND EXPLANATIONSarrow_forward
- give the structural formula for compounds A to Garrow_forward(a) Illustrate the following name reactions by giving example :(i) Cannizzaro’s reaction(ii) Clemmensen reduction(b) An organic compound A contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acids. Derive the possible structure of compound A.arrow_forwardA student was given from the list of the compounds below A, B and D blindly and asked to identify them all. He treated each of them with Brady's reagent (2,4-ditrophenylhydrazine) and isolated a bright yellow compound for one of them, but the other two gave false negatives. The student reasoned that the false negatives may be due to sterics and, on further thinking, it dawned on him that he might be able to rule out one of the false negatives with the haloform test. What compound did he find compatible with the haloform test? That compound did indeed give a false negative in the Brady test. Which of the other two was positive in the Brady test? A = haloform B = Brady A = haloform D = Brady B = haloform A = Brady B = haloform D = Brady D = haloform A = Brady D = haloform B = Bradyarrow_forward
- Draw the products formed by the following acid-base reaction without consideration of the equilibrium position: You do not have to consider stereochemistry. You should include all products. Include counter-ions, e.g., Na+, I-, in your submission, but draw them in their own separate sketcher. Separate structures with + signs from the drop-down menu.arrow_forward2 Provide the structure(s) of the expected major organic product(s) (where appropriate) and provide a detailed, reasonable, arrow-pushing mechanism. Hint: In the presence of a-keto acids, NR3 selectively reacts with the keto motif - not the carboxylic acid end.arrow_forwardComplete the following reactions by drawing the substrate, the products (majorities only) or by writing the reactional conditions. Take into account the stereochemistry.arrow_forward
- Please don't provide handwritten solution .... What is the major organic product from the following reaction?arrow_forward1) How will you describe whether any compound has been oxidized or reduced? Support the answer with suitable examples. 2)Why carboxylic acid with a carbonyl group at 3rd position can be decarboxylated? 3) Explain why electrophilic aromatic substitution in Pyrrole takes place at C-2 positions whereas, in Pyridine it takes place at C-3 position? 4) List the following esters in order of decreasing reactivities towards hydrolysis with reason: Methyl benzoate, p-cyano methyl benzoate and p-hydroxy methyl benzoate 5)LDA is the base of choice for carbonyl compound to completely convert into enolate. Why?arrow_forwardAs per the guidelines, three sub-parts will be answered, please help me with these three parts j, k lloudon 6th edition organic chemistry For each of the following reactions,provide the major product(s) or the necessary reagents to perform the chemical transformations indicated. If no reaction takes place, write NRarrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
