
(a)
Interpretation:
The synthesis of the given product from pyridine is to be predicted.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any
Answer to Problem 26.39AP
The synthesis for the given product from pyridine is shown below.
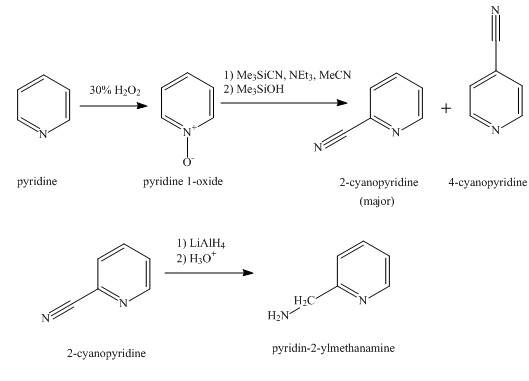
Explanation of Solution
In the first step, pyridine undergoes oxidation on nitrogen atom to form
In the second step, the treatment of
In the final step, the reduction of

Figure 1
The synthesis for the given product from pyridine is shown in Figure 1.
(b)
Interpretation:
The synthesis of the given product from pyridine is to be predicted.
Concept introduction:
The formation of diazonium salt from aromatic
The addition of the nitro group is known as nitration.
Answer to Problem 26.39AP
The synthesis for the given product from pyridine is shown below.
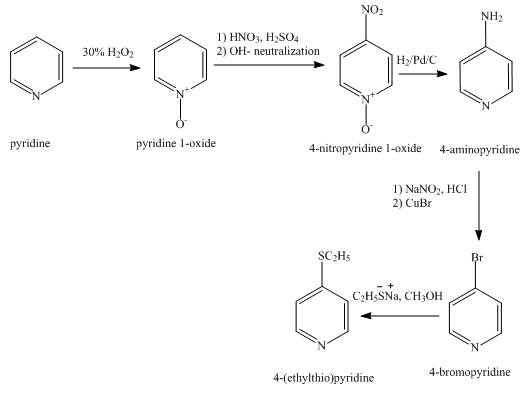
Explanation of Solution
In the first step, pyridine undergoes oxidation on nitrogen atom to form
In the third step, the reduction of
In the fourth step,
In the fifth step,
The overall reaction is shown below.

Figure 2
The synthesis for the given product from pyridine is shown in Figure 2.
(c)
Interpretation:
The synthesis of the given product from furfural is to be predicted.
Concept introduction:
Grignard reagents are
Answer to Problem 26.39AP
The synthesis of the given product from furfural is shown below.
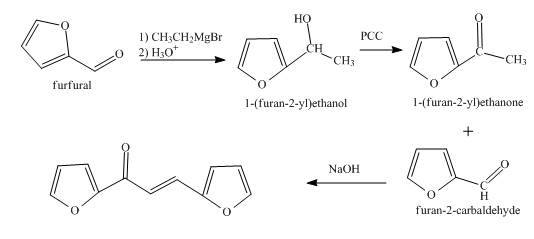
Explanation of Solution
In the first step, the treatment of fufural with
In the second step, the product obtained with the help of pyridinium chlorochromate undergoes oxidation to form the corresponding
In the final step, methyl ketone reacts with the furfural in the presence of
The overall reaction is shown below.

Figure 3
The synthesis of the given product from furfural is shown in Figure 3.
(d)
Interpretation:
The synthesis of the given product from furfural is to be predicted.
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 26.39AP
The synthesis of the given product from furfural is shown below.
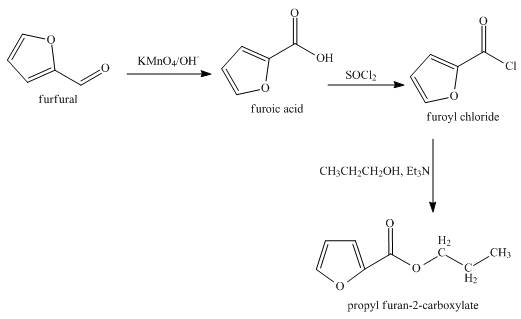
Explanation of Solution
In the first step, the reaction of furfural with
In the second step, furoic reacts acid with thionyl chloride to form furoyl chloride. The type of reaction is nucleophilic acyl substitution reaction.
In the third step, furoyl chloride with
The overall reaction is shown below.

Figure 4
The synthesis of the given product from furfural is shown in Figure 4.
(e)
Interpretation:
The synthesis of the given product from
Concept introduction:
Oxidation is the process of addition of oxygen. Oxidation is also defined as loss of electrons. In the oxidation process, there is increasing in the oxidation state. The acid is added in the reaction for protonation.
The oxidizing agent is defined as the species which oxidizes others and itself gets reduced.
Answer to Problem 26.39AP
The synthesis of the given product from
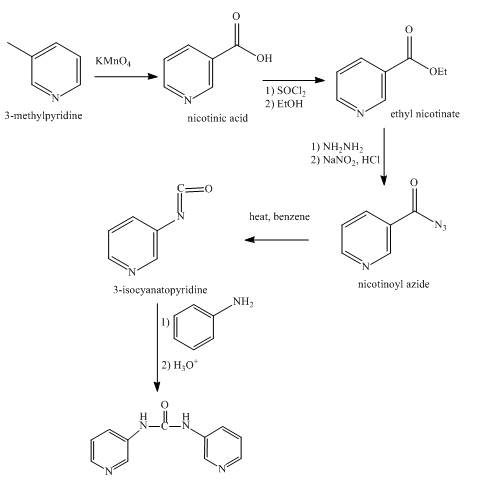
Explanation of Solution
In the first step,
In the second step, the reaction between nicotinic acid and thionyl chloride takes place in the presence of ethanol and gives the corresponding methyl ester as product.
In the third step, the reaction between methyl ester with hydrazine in the presence of
In the fourth step, the acyl azide on heating with benzene gives isocyanate as the product.
In the fifth step, the isocyanate is treated with

Figure 5
The synthesis of the given product from
(f)
Interpretation:
The synthesis of the given product from
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 26.39AP
The synthesis of the given product from
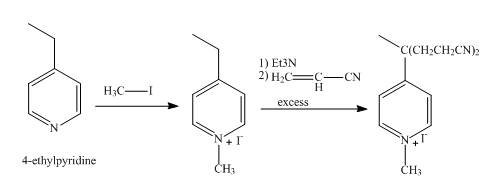
Explanation of Solution
In the first step, the reaction of
In the second step,
The overall reaction is shown below.

Figure 6
The synthesis of the given product from
(g)
Interpretation:
The synthesis of the given product from
Concept introduction:
The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as a substitution reaction. In a nucleophilic substitution reaction, nucleophile takes the position of leaving the group by attacking the electron-deficient carbon atom.
Answer to Problem 26.39AP
The synthesis of the given product from
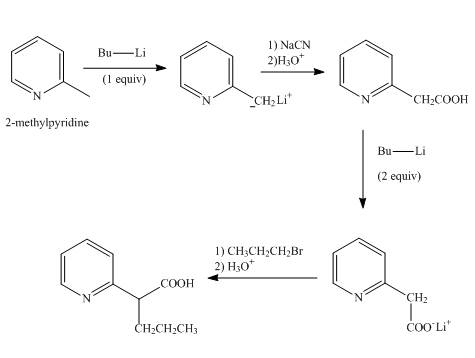
Explanation of Solution
In the first step,
In the second step, the treatment of resonance stabilized anion with sodium cyanide followed by hydrolysis gives a carboxylic acid product.
In the third step, the treatment of
In the final step, the resonance stabilized anion undergoes a nucleophilic substitution reaction with propyl bromide to form the desired product.
The overall reaction is shown below.

Figure 7
The synthesis of the given product from
Want to see more full solutions like this?
Chapter 26 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Outline the steps invloved in the synthesis of 3-chloro-4-fluroacetophenone from 4-aminoacetophenone. provide the bond line structure for the major organic product obtained in each step of the proposed synthesis.arrow_forwardOutline the synthesis of 2-pentyne from acetylene and any other needed organic or inorganic.arrow_forwardOutline the synthesis of the following from either malonic ester or acetoacetic ester or an enamine and using any other reagents of your choice:arrow_forward
- (a) suggest the suitable mechanism and illustrate the stepwise of the (heptyloxy)cyclopentane mechanism. (b) illustrate, and write the mechanism the formation of iodocyclopentane and 1-iodoheptane.arrow_forwardGive the structure of the product and/or intermediates of the following reactions. Indicate, whenappropriate, both regiochemistry and stereochemistryarrow_forwardthe following reaction scheme leads to the formation of compound C. give the structure of the final product C and of the intermediate products A and B and justify, using the mechanism, the formation of the product A. Give the serereochemistry of the final product obtainedarrow_forward
- Compound A was oxidized with periodic acid to give B, which after acid hydrolysis gave C. Bromine oxidation of C gave D. Suggest structural formulas, including stereochemistry, for compounds B, C, and D.arrow_forwardWhat happens when benzene and anisole react with sodium metal in the presence of ethanolic solution of ammonia. Also give mechanism for the reaction of benzene.arrow_forwardAssuming you are a chemist and you need to extract ethanolic acid in a benzene solution. Propose and briefly discuss how to extract the ethanolic acid in the benzene solution. Include the reactions involved in each step, if any.arrow_forward
- The synthesis of triphenylphosphine was carried out. Firstly the synthesis of the reagent, phenyl magnesium bromide followed by the addition of phosphorous tribromide which was further quenched, extracted and purified to give triphenylphosphine as a product. Write a reaction mechanism for this reaction. Discuss the reaction and what occurs.arrow_forwardorganic synthesis of Terephthalic acid from benzene, provide schemes/ figures of the synthesis structurearrow_forwardGive the structure of the product and/or intermediates of the following reactions. Indicate, where appropriate, both regiochemistry and stereochemistry.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
