
EBK ORGANIC CHEMISTRY
10th Edition
ISBN: 9781259636875
Author: Carey
Publisher: MCGRAW HILL BOOK COMPANY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 22P
(a) The two most acidic hydrogens of uracil have
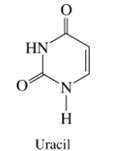
(b) The
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) The two most acidic hydrogens of uracil have pKa’s of 9.5 and 14.2, respectively. Match these pKa’s with the hydrogens in the structural formula and provide structures for the most stable resonance contributors of the monoanion and the dianion.(b) The pKa of the conjugate acid of triethylamine is 10.4. Is triethylamine a strong enough base to convert uracil to its monoanion? To its dianion?
Isoerythrogenic acid, C18H26O2, is a acetylic fatty acid that turns vivid vle on exposure to UV light. On Catalytic hydrogenation over a palladium catalyst, five molar equivalents of hydrogen are absorbed, and stearic acid
The pKa values of the carboxylic acid groups of oxaloacetic acid are 2.22 and 3.98.
a. Which carboxyl group is the stronger acid? b. The amount of hydrate present in an aqueous solution of oxaloacetic acid depends on the pH of the solution: 95% at pH 0, 81% at pH 1.3, 35% at pH 3.1, 13% at pH 4.7, 6% at pH 6.7, and 6% at pH 12.7. Explain this pH dependence.
Chapter 27 Solutions
EBK ORGANIC CHEMISTRY
Ch. 27.1 - Problem 27.1 Write a structural formula for the...Ch. 27.1 - Prob. 2PCh. 27.1 - Prob. 3PCh. 27.2 - Prob. 4PCh. 27.3 - Prob. 5PCh. 27.3 - Prob. 6PCh. 27.5 - Prob. 7PCh. 27.5 - Prob. 8PCh. 27.5 - Prob. 9PCh. 27.6 - Prob. 10P
Ch. 27.7 - Prob. 11PCh. 27.9 - Prob. 12PCh. 27.12 - 27.13 Modify Figure 27.12 so that it corresponds...Ch. 27.13 - Prob. 14PCh. 27 - Prob. 15PCh. 27 - Prob. 16PCh. 27 - Prob. 17PCh. 27 - Nebularine is a toxic nucleoside isolated from a...Ch. 27 - Prob. 19PCh. 27 - The 5-nucleotide of inosine, inosinic acid...Ch. 27 - Prob. 21PCh. 27 - (a) The two most acidic hydrogens of uracil have...Ch. 27 - The phosphorylation of -D-glucopyranose by ATP...Ch. 27 - When 6-chloropurine is heated with aqueous sodium...Ch. 27 - Prob. 25PCh. 27 - Prob. 26PCh. 27 - Prob. 27PCh. 27 - Prob. 28PCh. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...Ch. 27 - Oligonucleotide Synthesis In Section 27.6 we noted...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A chemically modified guanidino group is present in cimetidine (Tagamet), a widely prescribed drug for the control of gastric acidity and peptic ulcers. Cimetidine reduces gastric acid secretion by inhibiting the interaction of histamine with gastric H2 receptors. In the development of this drug, a cyano group was added to the substituted guanidino group to alter its basicity. Do you expect this modified guanidino group to be more basic or less basic than the guanidino group of arginine? Explain.arrow_forward1. Write an equation for the oxidation of glucose by CuO if the compound is oxidizedcompletely to CO2 and H2O. 2. What can you conclude regarding the difference in degree of acidity and alkalinity between organic and inorganic compounds? ( sample substances are dilute hcl, dilute acetic acid, dilute nh4oh and aniline)arrow_forwardThe pKa values of the carboxylic acid groups of oxaloacetic acid are 2.22 and 3.98.a. Which carboxyl group is the stronger acid?b. The amount of hydrate present in an aqueous solution of oxaloacetic acid depends on the pH of the solution: 95% at pH 0, 81% at pH 1.3, 35% at pH3.1, 13% at pH 4.7, 6% at pH 6.7, and 6% at pH 12.7. Explain this pH dependence.arrow_forward
- Tyrosine is an amino acid whose side chain has a pKa of 10.1. At pH 7, what protonation form would you expect to find it in?arrow_forwardThe compound acetophenone has a very similar molar mass to that of benzoic acid and benzamide. However, acetophenone has a much lower m.p. (20 °C) than both such that, by contrast, it is a liquid at room temperature. By considering intermolecular forces and comparing functional group structure, account for this big difference in physical properties.arrow_forward18-48 4-Aminobenzoic acid is prepared from benzoic acid by the following two steps. Show reagents and experimental conditions to bring about each step.arrow_forward
- 1 Write an equation for the hydrolysis of trimethyl phosphate to dimethyl phosphate and methanol in aqueous base. Show each product as it would be ionized in this solution.arrow_forwardAn organic compound is analysed and found to contain 66.7% carbon, 11.2% hydrogen and 22.1% oxygen by mass. The compound boils at 79.6 C. At 100 C and 0.970atm, the vapour has a density of 2.28g/L. The compound has a carbonyl group and cannot be oxidized to a carboxylic acid. Suggest a structure for the compound.arrow_forwardIf the pKa of the carboxylic acid groups on acetoacetic acid and B-hydroxybutyric acid are 3.6 and 4.7, respectively, which form (protonated or deprotonated) is present at physiological pH?arrow_forward
- Predict the reactions of lipids under basic hydrolysis and with standard organicreagents. Show the reactions of the ester and olefinic groups of glycerides and thecarboxyl groups of fatty acids.arrow_forwardThe isoelectric point (pl) of phenylalanine is pH 5.5. Draw the structure of the major form of glutamic acid at pH values of (a) 1 (b) 5.5 (c) 11.0.arrow_forwardExplain the effect of the pH on the protonation of the functional groups present in ascorbic acid (C6H8O6) and citric acid (C6H8O7).arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License