
ORG CHEM LL W/ LL SG&CONPLUS PKG>IC<
5th Edition
ISBN: 9781260069228
Author: SMITH
Publisher: MCG CUSTOM
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 27, Problem 27.25P
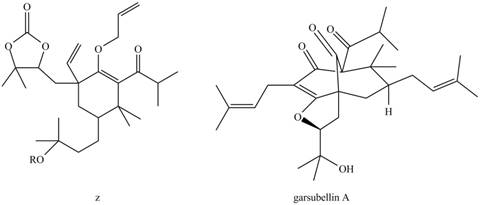
(a) What product is formed by the Claisen rearrangement of compound Z? (b) Using what you have learned about ring-closing metathesis in Chapter 26, draw the product formed when the product in part (a) is treated with Grubbs catalyst. These two reactions are key steps in the synthesis of garsubellin A, a biologically active natural product that stimulates the synthesis of the neurotransmitter acetylcholine. Compounds of this sort may prove to be useful drugs for the treatment of neurodegenerative diseases such as Alzheimer’s disease.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
a) Which base would be the best base to use for both of these and why?
b) Give the expected product for the Claisen condensation reaction of A).
c) Compound A) will successfully undergo a Claisen condensation reaction, however, Compound B) will not. Briefly explain why, and show the form of the product for Compound A) that makes the reaction possible.
Drawing an SN2 product with More Complex Reactants
Identify C, the product of an SN2 reaction in the synthesis of raloxifene, a drug used to reduce the risk of invasive breast cancer in postmenopausal women.
please help with this question. thank you.
The following sequence, beginning with a cyclic hemiacetal (compound A), was part of a recently reported enantiospecific synthesis of a powerful sex pheromone (currently used in pest management) of the mealybug Pseudococcus viburni:
Draw the structures of compound B and C. Provide a plausible mechanism to explain the transformation from compound C into compound D. Identify the reagents you would need to convert compound D into compound F (in just two steps). Also identify the structure of compound E.
Chapter 27 Solutions
ORG CHEM LL W/ LL SG&CONPLUS PKG>IC<
Ch. 27 - Prob. 27.1PCh. 27 - Problem 27.2
For each molecular orbital in Figure...Ch. 27 - Problem 27.3
(a) Using Figure 27.2 as a guide,...Ch. 27 - Problem 27.4
(a) How many molecular orbitals are...Ch. 27 - Prob. 27.5PCh. 27 - Prob. 27.6PCh. 27 - Prob. 27.7PCh. 27 - Prob. 27.8PCh. 27 - Prob. 27.9PCh. 27 - Prob. 27.10P
Ch. 27 - Problem 27.11
What product would be formed by the...Ch. 27 - Consider cycloheptatrienone and ethylene, and draw...Ch. 27 - Problem 27.13
Show that a thermal suprafacial...Ch. 27 - Prob. 27.14PCh. 27 - a Draw the product of the following [4+2]...Ch. 27 - Prob. 27.16PCh. 27 - Prob. 27.17PCh. 27 - Problem 27.18
Using orbital symmetry, explain why...Ch. 27 - Prob. 27.19PCh. 27 - Prob. 27.20PCh. 27 - Prob. 27.21PCh. 27 - Prob. 27.22PCh. 27 - Prob. 27.23PCh. 27 - Prob. 27.24PCh. 27 - Problem 27.25
(a) What product is formed by the...Ch. 27 - Prob. 27.26PCh. 27 - Prob. 27.27PCh. 27 - Prob. 27.28PCh. 27 - Prob. 27.29PCh. 27 - Prob. 27.30PCh. 27 - Prob. 27.31PCh. 27 - Prob. 27.32PCh. 27 - Prob. 27.33PCh. 27 - Prob. 27.34PCh. 27 - Prob. 27.35PCh. 27 - Prob. 27.36PCh. 27 - Prob. 27.37PCh. 27 - Prob. 27.38PCh. 27 - Prob. 27.39PCh. 27 - Prob. 27.40PCh. 27 - 27.41 What starting materials are needed to...Ch. 27 - Prob. 27.42PCh. 27 - Prob. 27.43PCh. 27 - Prob. 27.44PCh. 27 - Prob. 27.45PCh. 27 - Prob. 27.46PCh. 27 - 27.47 What product is formed from the [5,5]...Ch. 27 - Prob. 27.48PCh. 27 - 27.49 Draw structures for A, B, and C in the...Ch. 27 - Prob. 27.50PCh. 27 - Prob. 27.51PCh. 27 - 27.52 Draw the products of each reaction.
c....Ch. 27 - Prob. 27.53PCh. 27 - 27.54 Draw a stepwise, detailed mechanism for the...Ch. 27 - Prob. 27.55PCh. 27 - Prob. 27.56PCh. 27 - Prob. 27.57PCh. 27 - 27.58 Draw a stepwise, detailed mechanism for the...Ch. 27 - Prob. 27.59PCh. 27 - Prob. 27.60PCh. 27 - Prob. 27.61PCh. 27 - Prob. 27.62PCh. 27 - Prob. 27.63P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Tamoxifen is an estrogen receptor modulator that is used in the treatment of breast cancer. Provide the missing reagents and the structure of compound A in the synthesis of tamoxifen.arrow_forwardA. Arrange the following radicals in order of decreasing rate of bromination. Justify your answer. B. Trehalose and isomaltose are both dimers of glucose. However, they have considerablydifferent reactivities. Concisely explain why these differences are observed. -Isomaltose is a reducing sugar while trehalose is not.-Trehalose is very resistant to acid hydrolysis while isomaltose can be acid-hydrolyzed withease.arrow_forwardWhen benzene is treated with methyl chloride and Aluminum chloride under conditions that favor trialkylation, one major product is obtained. Draw the product and provide a name for the product.arrow_forward
- A key step in the synthesis of β-vetivone, a major constituent of vetiver, a perennial grass found in tropical and subtropical regions of the world, involved the reaction of compound A and dihalide B with two equivalents of LDA to form C. Draw a stepwise mechanism for this reaction. β-Vetivone contains a spiro ring system—that is, two rings that share a single carbon atom.arrow_forwardDraw the reaction scheme for AlCl3 was dissolved in acetyl chloride and cooled in an ice/water bath. 1,3,5-Triphenylbenzene was dissolved in CH2Cl2, and gradually added to the cold AlCl3/acetyl chloride solution.arrow_forwardCompound A is first reacted with methylamine in the presence of acid and then treated with NaBH3CN. Using the spectroscopic data given, what is the structure of the product after step 1?arrow_forward
- (−)-Hyoscyamine, an optically active drug used to treat gastrointestinal disorders, is isolated from Atropa belladonna, the deadly nightshade plant, by a basic aqueous extraction procedure. If too much base is used during isolation, optically inactive material is isolated. (a) Explain this result by drawing a stepwise mechanism. (b) Explain why littorine, an isomer isolated from the tailflower plant in Australia, can be obtained optically pure regardless of the amount of base used during isolation.arrow_forwardWhen a single compound contains both a nucleophile and a leaving group, an intramolecular reaction occurs. With this in mind, what is the structure and stereochemistry of the following reaction?arrow_forwardDraw the product of this reaction and states its IUPAC name, and states what type of mechanism is occuring (eg. SN1, SN2, E1, E2, etc)?arrow_forward
- Friedel–Crafts alkylation of benzene with (R)-2-chlorobutane and AlCl3 affords sec-butylbenzene.a. How many stereogenic centers are present in the product?b. Would you expect the product to exhibit optical activity? Explain, with reference to the mechanism.arrow_forwardDescribe the following chemical reactions as SN1, SN2, E1 and W2. Draw a curved arrow mechanism for each reaction.arrow_forwardThe compound below is treated with chlorine in the presence of lightarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Characteristic Reactions of Benzene and Phenols; Author: Linda Hanson;https://www.youtube.com/watch?v=tjEqEjDd87E;License: Standard YouTube License, CC-BY
An Overview of Aldehydes and Ketones: Crash Course Organic Chemistry #27; Author: Crash Course;https://www.youtube.com/watch?v=-fBPX-4kFlw;License: Standard Youtube License