
Concept explainers
(a)
The ground state energy of the electron.
(a)
Answer to Problem 55P
The ground state energy of the electron is
Explanation of Solution
The energy of the photon emitted during transition from the first excited state to the ground state is
Write the expression for energy of the quantized level in terms of the ground state energy.
Here,
Substituting
Here,
Write the expression for energy difference between the first excited state and the ground state.
Here,
Substituting (II) in (III)
Rearranging (IV)
Substituting
Thus, the ground state energy is
(b)
The possible energies of the emitted photon if the electron is initially in the third excited state.
(b)
Answer to Problem 55P
The possible energies of the emitted photon in the increasing order are
Explanation of Solution
If the initial state of the electron is
Substitute
Subtracting the corresponding energies in the allowed transition to find the energy of the photon and substitute
For
For
For
For
For
For
Thus, the possible energies of the emitted photon in the increasing order are
(c)
The plot of the wave function of the electron in the third excited state.
(c)
Answer to Problem 55P
The wave function is a sign wave with three nodes.
Explanation of Solution
Write the expression for the wave function of a particle in a box.
Here,
Substitute
The plot of the wave function versus position is given below:
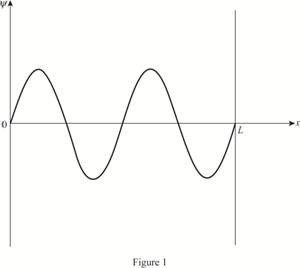
(d)
The effect of increasing the length of the box on its energy.
(d)
Answer to Problem 55P
The energy spacing between two levels decreases.
Explanation of Solution
Write the expression for the energy of a particle in a box.
Here,
Want to see more full solutions like this?
Chapter 28 Solutions
Student Solutions Manual for Physics
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





