
Concept explainers
(a)
The length of the one-dimensional box.
(a)
Answer to Problem 72P
The length of the box is
Explanation of Solution
The ground state energy of the electron in a box is
Write the expression for energy of the quantized level.
Here,
Write the expression for ground state energy for a particle in box.
Here,
Rearranging (I)
Substituting
Thus, the length of the box is
(b)
The plot of wave functions of the first three state.
(b)
Explanation of Solution
Write the expression for the wave function of a particle in a box.
Here,
Substitute
Here,
Here,
Here,
The plot of the wave functions versus position is given below:
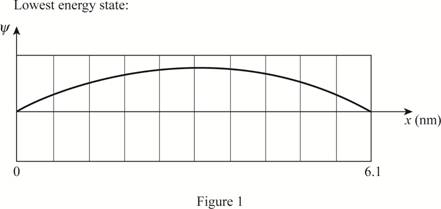
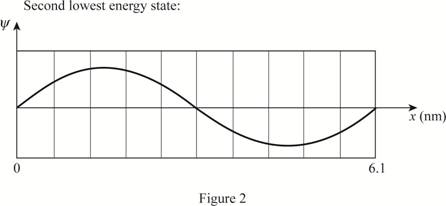
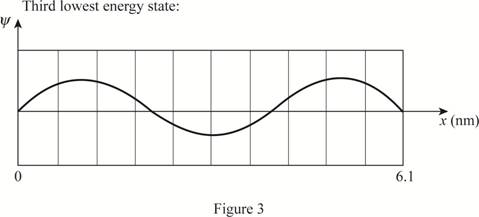
(c)
The wavelength of the electron in the third state.
(c)
Answer to Problem 72P
The wave length of the electron in the second excited state is
Explanation of Solution
Substituting
Here,
Write the expression for de Broglie wavelength of an electron.
Here,
The particle in a box model renders only the kinetic energy since the potential is zero at the box and is infinite everywhere else. Thus, consider,
Substituting
Rearranging
Substituting
Thus, the wave length of the electron in the second excited state is
(d)
The wavelength of the emitted photon during the downward transition of the electron.
(d)
Answer to Problem 72P
The possible wavelengths of the emitted photons are
Explanation of Solution
The wavelength of the photon is
Write the expression for energy of a photon.
Here,
Write the expression for energy of the quantized levels in terms of the ground state energy
Subtracting
Here,
Rearranging (XII)
Substituting
Substitute
Thus when the electron absorbs the photon, it is excited to the third state.
The possible downward transitions are
Write the expression for energy of the photon during a downward transition.
Here,
Substituting
Substituting
Substituting
For,
Substituting
Substituting
For,
For,
Thus, the possible wavelengths of the emitted photons are
Want to see more full solutions like this?
Chapter 28 Solutions
Student Solutions Manual for Physics
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON





