
ORGANIC CHEMISTRY-EBOOK>I<
9th Edition
ISBN: 9781305084414
Author: McMurry
Publisher: INTER CENG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 28.SE, Problem 16MP
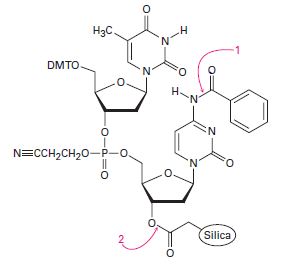
The final step in DNA synthesis is deprotection by treatment with aqueous ammonia. Show the mechanisms by which deprotection occurs at the points indicated in the following structure:
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Identify the organic functional group and reaction type for the following reaction.
The reactant is a(n)
- carboxylic acid hexose
- Aldohexose
- aldotetrose
-deoxyhexose
-carboxylic acid tetrose
- ketohexose
The product is a(n)
- carboxylic acid tetrose
- aldotetrose
-alcohol hexose
-aldohexose
-carboxylic acid hexose
- alcohol tetrose
The reaction type is
- hemiacetal formation
-hydrolysis
-oxidation( Benedict’s)
-acetal formation
-reduction( hydrogenation)
- mutarotation
An octapeptide contains the following amino acids: Arg, Glu, His, Ile, Leu, Phe, Tyr, and Val. Carboxypeptidase treatment of the octapeptide forms Phe and a heptapeptide. Treatment of the octapeptide with chymotrypsin forms two tetrapeptides, A and B. Treatment of A with trypsin yields two dipeptides, C and D. Edman degradation cleaves the following amino acids from each peptide: Glu (octapeptide), Glu (A), Ile (B), Glu (C), and Val (D). Partial hydrolysis of tetrapeptide B forms Ile–Leu in addition to other products. Deduce the structure of the octapeptide and fragments A–D.
Complete the structures of these two am ino acids at the pH values given.
Explain why the structures change at different pH values.
ÇH3
H2N-
-COOH
H2N-C
СООН
H
H
alanine
glycine
at pH 1
at pH 7
at pH 14
Chapter 28 Solutions
ORGANIC CHEMISTRY-EBOOK>I<
Ch. 28.1 - Draw the full structure of the DNA dinucleotide...Ch. 28.1 - Draw the full structure of the RNA dinucleotide...Ch. 28.2 - What sequence of bases on one strand of DNA is...Ch. 28.4 - Show how uracil can form strong hydrogen bonds to...Ch. 28.4 - What RNA base sequence is complementary to the...Ch. 28.4 - From what DNA base sequence was the following RNA...Ch. 28.5 - List codon sequences for the following amino...Ch. 28.5 - List anticodon sequences on the tRNAs carrying the...Ch. 28.5 - What amino acid sequence is coded by the following...Ch. 28.5 - What is the base sequence in the original DNA...
Ch. 28.7 - Prob. 11PCh. 28.7 - Prob. 12PCh. 28.SE - Identify the following bases, and tell whether...Ch. 28.SE - Identify the following nucleotide, and tell how it...Ch. 28.SE - Amine bases in nucleic acids can react with...Ch. 28.SE - The final step in DNA synthesis is deprotection by...Ch. 28.SE - The final step in the metabolic degradation of...Ch. 28.SE - One of the steps in the biosynthesis of a...Ch. 28.SE - One of the steps in the metabolic degradation of...Ch. 28.SE - One of the steps in the biosynthesis of uridine...Ch. 28.SE - Human brain natriuretic peptide (BNP) is a small...Ch. 28.SE - Human and horse insulin both have two polypeptide...Ch. 28.SE - The DNA of sea urchins contains about 32% A. What...Ch. 28.SE - The codon UAA stops protein synthesis. Why does...Ch. 28.SE - Which of the following base sequences would most...Ch. 28.SE - For what amino acids do the following...Ch. 28.SE - Prob. 27APCh. 28.SE - Prob. 28APCh. 28.SE - Draw the complete structure of the ribonucleotide...Ch. 28.SE - Draw the complete structure of the...Ch. 28.SE - Give an mRNA sequence that will code for the...Ch. 28.SE - Give an mRNA sequence that will code for the...Ch. 28.SE - What amino acid sequence is coded for by the...Ch. 28.SE - What amino acid sequence is coded for by the...Ch. 28.SE - Prob. 35APCh. 28.SE - Show the steps involved in a laboratory synthesis...Ch. 28.SE - Draw the structure of cyclic adenosine...Ch. 28.SE - Prob. 38AP
Additional Science Textbook Solutions
Find more solutions based on key concepts
For each of the following 2-dimensional shapes, determine the highest order rotation axis of symmetry.
Inorganic Chemistry
Which of the following solutions has the higher molarity? 10 ppm KI in water or 10,000 ppb KBr in water 0.25 ma...
CHEMISTRY-TEXT
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry
4.1 Write the symbols for the following elements.
a. copper
b. platinum
c. calcium
d. manganese
e. Iron
...
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Excess ascorbic acid is excreted in the urine, the pH of which is normally in the range 4.8–8.4. What form of ascorbic acid would you expect to be present in urine of pH 8.4— free ascorbic acid or ascorbate anion? Explainarrow_forward(a) What is the difference between the hormones progesterone and testosterone? (b) Draw the structure of a a steroid nucleus. (c) Give the products obtained from complete base hydrolysis in the following reaction: O || CH,−O−C−(CH2)14–CH3 O CH–0–C−(CH2)14—CH3 + 3 NaOH O CH,−0–C−(CH2)14–CH3arrow_forwardO alanine glycine Ovaline Oleucine O isoleucine HN Leucine Alanine 1. Excess NH,+ trace acid 3. HC, H₂O + heat Glyche Alm Mis holmainearrow_forward
- The first step in the catabolism of most amino acids is the removal of the nitrogen atom by transfer to an a-keto acid, a reaction catalyzed by an enzyme called a transaminase. The a-keto acid acceptor is often a-ketoglutarate. Modify the structures in the product to show the products of the transamination of cysteine. Be sure to show functional groups with the charge and number of attached hydrogen atoms appropriate for pH 7.4. transaminase + O=C H₂N-CH + CH₂ CH₂ CH₂ SH Incorrect H₂N || CH | CH₂ | CH₂ I || O || n | CH₂ T SHarrow_forwardThe dynorphins are a group of opioid peptides that play an importantrole in changes in the brain associated with cocaine addiction. One ofthese peptides, dynorphin A, contains the following amino acidsequence: Tyr–Gly–Gly–Phe–Leu–Arg–Arg–Ile–Arg–Pro–Lys–Leu–Lys.Draw the amino acids and peptide fragments formed when dynorphin A is treated with each reagent or enzyme: (a) chymotrypsin; (b) trypsin; (c)carboxypeptidase; (d) C6H5N=C=S.arrow_forwardA variation of the acetamidomalonate synthesis can be used to synthesize serine. The process involves the following steps: Ethoxide ion deprotonates diethyl acetamidomalonate, forming enolate anion 1; Enolate anion 1 makes a nucleophilic attack on formaldehyde, forming tetrahedral intermediate 2; Protonation of the oxyanion forms alcohol 3; Acid hydrolysis yields dicarboxyamino alcohol 4; Decarboxylation leads to the final amino acid. Write out the mechanism on a separate sheet of paper, and then draw the structure of tetrahedral intermediate 2. You do not have to consider stereochemistry. You do not have to explicitly draw H atoms. Do not include lone pairs in your answer. They will not be considered in the grading. Draw carboxyl and amino groups in their uncharged forms.arrow_forward
- Draw the structure of the predominant form of a mixture of alanine, lysine, and aspartic acid at (i) pH 6; (ii) pH 11; (iii) pH 2.arrow_forwardMany multi-step organic reaction mechanisms involve proton transfer steps. For example, the first step of Fischer esterification of carboxylic acids(as shown with acedic acid) is activation of the acid by protonation. Based on your understanding of which reaction pathway is more favorable, explain why using chemical structures.arrow_forward3a. 3b. 3c 3d. 3e. CO₂ clavulanic acid CH₂-OH H Answer the following questions about clavulanic acid. Does clavulanic acid inhibit D-alanyl-D-alanine transpeptidase? Does clavulanic acid contain a ß-lactam? Does clavulanic acid contain a thiazolium ring? What is the result of the treatment of penicillinase with clavulanic acid? Does clavulanic acid form a covalent acyl-enzyme intermediate with penicillinase?arrow_forward
- 6. P2O5 is an anhydride of НРОЗ НЗРО4 НЗРОЗ H2P207arrow_forwardEthyleneimine reacts with cysteine side chains in proteins to form S - aminoethyl derivatives. The peptide bonds on the carboxyl side of these modified cysteine residues are susceptible to hydrolysis by trypsin . Why?arrow_forwardThe Ka1 of ascorbic acid is 7.94 x 10-5. Would you expect ascorbic acid dissolved in blood plasma (pH 7.35–7.45) to exist primarily as ascorbic acid or as ascorbate anion? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning
Nucleic acids - DNA and RNA structure; Author: MEDSimplified;https://www.youtube.com/watch?v=0lZRAShqft0;License: Standard YouTube License, CC-BY