
Concept explainers
Draw the product formed when the following amino acid is treated with each reagent:
(a)
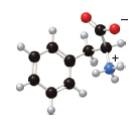
(a)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 29.28P
The products formed by the treatment of given amino acid with
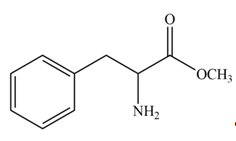
Explanation of Solution
In the given ball-and-stick model of amino acid, the black balls represent carbon atoms, the white balls represent hydrogen atoms, the blue ball represents nitrogen atom and the red balls represent oxygen atoms. Therefore, the structure of given amino acid is,

Figure 1
The protection of carboxyl group takes place on reaction with
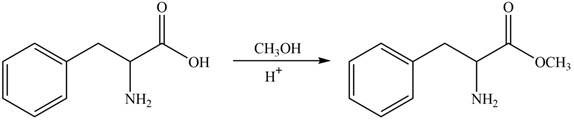
Figure 2
The products formed by the treatment of given amino acid with
(b)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 29.28P
The products formed by the treatment of given amino acid with
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
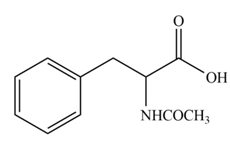
The amine group of amino acid form amide bond on reaction with acid chloride as shown in Figure 3.
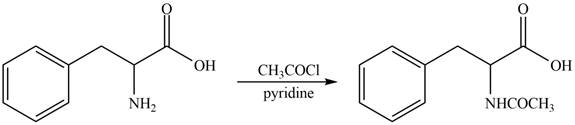
Figure 3
The products formed by the treatment of given amino acid with
(c)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 29.28P
The products formed by the treatment of given amino acid with
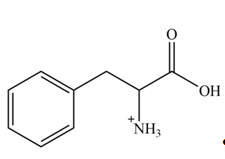
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
The amine group of amino acid form amide bond on reaction with acid chloride as shown in Figure 4.
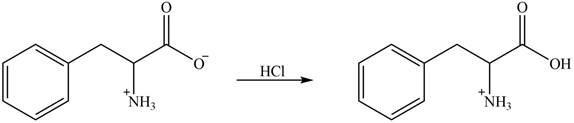
Figure 4
The products formed by the treatment of given amino acid with
(d)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 29.28P
The products formed by the treatment of given amino acid with
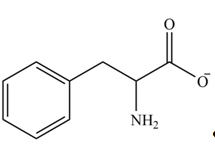
Explanation of Solution
The structure of given amino acid is shown in Figure 1.
The amine group of amino acid form amide bond on reaction with acid chloride as shown in Figure 5.
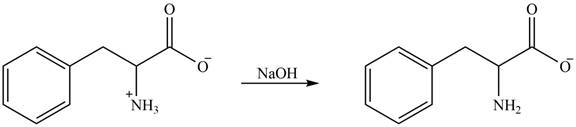
Figure 5
The products formed by the treatment of given amino acid with
(e)
Interpretation: The products formed by the treatment of given amino acid with
Concept introduction: The chemical compounds in which carbon is bonded with acidic and basic group along with hydrocarbon side chain are known as amino acids. The amine and carboxyl group of amino acid shows different reactions with alcohols, acid chloride, acid and base.
Answer to Problem 29.28P
The products formed by the treatment of given amino acid with
Explanation of Solution
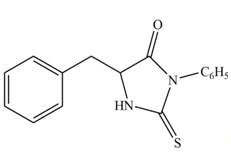
The structure of given amino acid is shown in Figure 1.
The terminal amine group of amino acid on reaction with phenyl isothiocyanate form N-phenylthiohydantoin as shown in Figure 6.
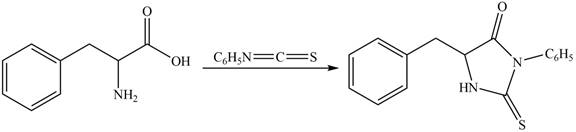
Figure 6
The products formed by the treatment of given amino acid with
Want to see more full solutions like this?
Chapter 29 Solutions
Organic Chemistry
- 22-59 What is the effect of salt bridges on the tertiary structure of proteins?arrow_forward22-48 How many amino acid residues in the A chain of insulin are the same in insulin from humans, cattle (bovine), hogs, and sheep?arrow_forward22-42 (a) How many atoms of the peptide bond lie in the same plane? (b) Which atoms are they?arrow_forward
- Draw a representative amino acid, labelling the side group as R and identifying the amino and carboxylic acid groups.arrow_forwardDraw the predominant form the glutamine amino acids at physiological pH (7.4):arrow_forwardThe amino acid alanine is a solid at room temperature and has a melting point of 315 °C, while pyruvic acid (CH 3COCO 2H) has a similar molecular weight but is a liquid at room temperature with a boiling point of 165 °C. Account for the difference.arrow_forward
- The first amino acid incorporated into a polypeptide chain during its biosynthesis in prokaryotes is N-formylmethionine. Explain the purpose of the formyl grouparrow_forwardalpha-Amino acids can be prepared by treating an aldehyde with ammonia/trace acid, followed by hydrogen cyanide, followed by acid-catalyzed hydrolysis. a. Draw the structures of the two intermediates formed in this reaction. b. What amino acid is formed when the aldehyde that is used is 3-methylbutanal? c. What aldehyde is needed to prepare isoleucine?arrow_forwardThe dynorphins are a group of opioid peptides that play an importantrole in changes in the brain associated with cocaine addiction. One ofthese peptides, dynorphin A, contains the following amino acidsequence: Tyr–Gly–Gly–Phe–Leu–Arg–Arg–Ile–Arg–Pro–Lys–Leu–Lys.Draw the amino acids and peptide fragments formed when dynorphin A is treated with each reagent or enzyme: (a) chymotrypsin; (b) trypsin; (c)carboxypeptidase; (d) C6H5N=C=S.arrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning




