
Concept explainers
What is the predominant form of each of the following amino acids at
(a)
Interpretation: The predominant form of threonine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of threonine at
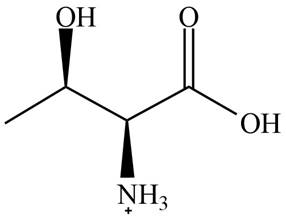
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of threonine is
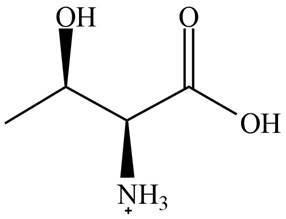
Figure 1
The overall charge on threonine at
The predominant form of threonine at
(b)
Interpretation: The predominant form of methionine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of methionine at
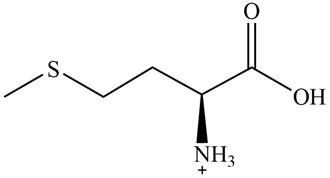
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of methionine is
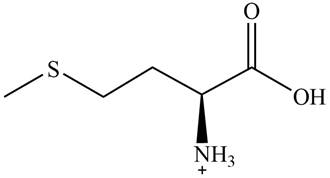
Figure 2
The overall charge on methionine at
The predominant form of methionine at
(c)
Interpretation: The predominant form of aspartic acid at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of aspartic acid at
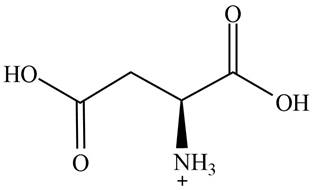
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of aspartic acid is
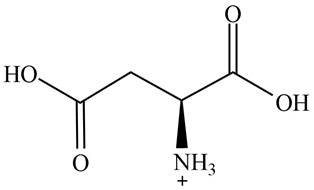
Figure 3
The overall charge on aspartic acid at
The predominant form of aspartic acid at
(d)
Interpretation: The predominant form of arginine at
Concept introduction: At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
Answer to Problem 29.37P
The predominant form of arginine at
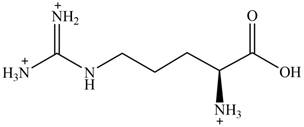
The overall charge on it is
Explanation of Solution
At isoelectric point, the amino acids exist in their neutral form. The amine groups exists as
The isoelectric point of arginine is
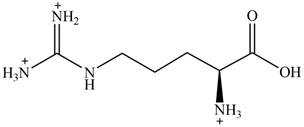
Figure 4
The overall charge on arginine at
The predominant form of arginine at
Want to see more full solutions like this?
Chapter 29 Solutions
Organic Chemistry
- How many of the -amino acids shown in Table 26-1 contain aromatic rings? How many contain sulfur? How many contain alcohols? How many contain hydrocarbon side chains?arrow_forwardAn amino acid residue side chain is deprontonated at pH9.5. What is the pKa of this residue if it is fully deprotonated at this pH?arrow_forwardAt very low pH, alanine is a diprotic acid that can be represented as H3N1-CH(CH3)-COOH. The pKa of the carboxyl group is 2.3, and the pKa of the UNH1 3 group is 9.7.(a) At pH 7, what fraction of the amino acid molecules dissolved in an aqueous solution will have the form H3N1-CH(CH3)-COO2?(b) What fraction of the molecules at this pH will havethe form H2N-CH(CH3)-COOH?arrow_forward
- Treatment of a new protein with dansyl chloride reveals two (2) dansyl-labelled derivatives of amino acids, alanine and methionine. What can you deduce about the structure of the proteinfrom these results?arrow_forwardDraw the structure of the tetrapeptide Asp-Arg-Val-Tyr. Please show the appropriate stereochemistry of the natural amino acids in the resulting peptide. Please draw all ionizable groups in their neutral form.arrow_forwardThe pKa of the amino and carboxyl groups on alanine are 9.9 and 2.4, respectively. Under physiological conditions (pH 7.4), what percentage of alanine is ionized?arrow_forward
- What is the amino terminal residue? What is the carboxyl terminal residue? How many amino acid residues are in the peptide? The net charge of this peptide at pH 7.4 would be?arrow_forwardIf you were to design a small peptide with a large net negative charge at physiological pH, which amino acid residues should predominate?arrow_forwardWhat is the predominant form of the following amino acids at pH= 1? What is the overall charge on the amino acid at this pH? aspartic acidarrow_forward
- Which of the following amino acids has a side chain with an ionizable proton?arrow_forwardWhich amino acid has the greatest amount of negative charge at pH = 6.20?arrow_forwardA peptide has the sequence Glu–His–Trp–Ser–Gly–Leu–Arg–Pro–Gly The p?a values for the peptide’s side chains, terminal amino groups, and carboxyl groups are provided in the table. Amino acid Amino pKa Carboxyl pKa Side‑chain pKa glutamate 9.609.60 2.342.34 4.254.25 histidine 9.179.17 1.821.82 6.006.00 tryptophan 9.399.39 2.382.38 serine 9.159.15 2.212.21 glycine 9.609.60 2.342.34 leucine 9.609.60 2.362.36 arginine 9.049.04 2.172.17 12.4812.48 proline 10.9610.96 1.991.99 Calculate the net charge of the molecule at pH 11 and estimate the isoelectric point (pI)(pI) for this peptide.arrow_forward
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



