
Organic Chemistry
4th Edition
ISBN: 9780073402772
Author: Janice G. Smith
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 29, Problem 29.29P
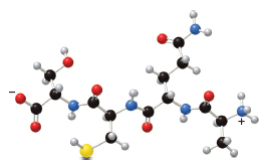
With reference to the following peptide: (a) Identify the
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(A) How many peptide bonds are present in peptide 1?
(B) What is the N-terminal amino acid in peptide 2?
(C) What is the C-terminal amino acid in peptide 2?
(a) Draw the structure of the two possible dipeptides that can be formed by combining valine and phenylalanine. (b) In each dipeptide, label the N- and C-terminal amino acids. (c) Name each peptide using three-letter symbols.
Calculate the attraction between an arginine and glutamate at pH 7 under thefollowing conditions:a) in air, spaced 5 Å apartb) in water, spaced 5 Å apartc) in ethanol, spaced 5 Å apart
Chapter 29 Solutions
Organic Chemistry
Ch. 29 - Prob. 29.1PCh. 29 - Problem 29.2
What form exists at the isoelectric...Ch. 29 - Problem 29.3
Explain why the of the group of an...Ch. 29 - Problem 29.5
What -halo carbonyl compound is...Ch. 29 - Problem 29.6
The enolate derived from diethyl...Ch. 29 - Problem 29.7
What amino acid is formed when is...Ch. 29 - Problem 29.8
What aldehyde is needed to synthesize...Ch. 29 - Prob. 29.8PCh. 29 - Prob. 29.9PCh. 29 - Prob. 29.10P
Ch. 29 - Prob. 29.11PCh. 29 - Problem 29.13
What alkene is needed to synthesize...Ch. 29 - Problem 29.14
Draw the structure of each peptide....Ch. 29 - Name each peptide using both the one-letter and...Ch. 29 - Prob. 29.15PCh. 29 - Prob. 29.16PCh. 29 - Problem 29.18
Glutathione, a powerful antioxidant...Ch. 29 - Problem 29.19
Draw the structure of the...Ch. 29 - Problem 29.20
Give the amino acid sequence of an...Ch. 29 - a What products are formed when each peptide is...Ch. 29 - Prob. 29.21PCh. 29 - Devise a synthesis of each peptide from amino acid...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.24PCh. 29 - Consider two molecules of a tetrapeptide composed...Ch. 29 - What types of stabilizing interactions exist...Ch. 29 - Prob. 29.27PCh. 29 - Draw the product formed when the following amino...Ch. 29 - With reference to the following peptide: a...Ch. 29 - Devise a synthesis of the following dipeptide from...Ch. 29 - Prob. 29.31PCh. 29 - Prob. 29.32PCh. 29 - Histidine is classified as a basic amino acid...Ch. 29 - Tryptophan is not classified as a basic amino acid...Ch. 29 - What is the structure of each amino acid at its...Ch. 29 - To calculate the isoelectric point of amino acids...Ch. 29 - What is the predominant form of each of the...Ch. 29 - 29.37 What is the predominant form of each of the...Ch. 29 - a. Draw the structure of the tripeptide A–A–A, and...Ch. 29 - Draw the organic product formed when the amino...Ch. 29 - 29.39 Draw the organic products formed in each...Ch. 29 - 29.40 What alkyl halide is needed to synthesize...Ch. 29 - 29.41 Devise a synthesis of threonine from diethyl...Ch. 29 - 29.42 Devise a synthesis of each amino acid from...Ch. 29 - Prob. 29.45PCh. 29 - Prob. 29.46PCh. 29 - Prob. 29.47PCh. 29 - Prob. 29.48PCh. 29 - Prob. 29.49PCh. 29 - 29.48 Brucine is a poisonous alkaloid obtained...Ch. 29 - Prob. 29.51PCh. 29 - Prob. 29.52PCh. 29 - Draw the structure for each peptide: (a) Phe–Ala;...Ch. 29 - 29.52 For the tetrapeptide Asp–Arg–Val–Tyr:
a....Ch. 29 - Prob. 29.55PCh. 29 - Explain why a peptide CN bond is stronger than an...Ch. 29 - Prob. 29.57PCh. 29 - 29.55 Draw the amino acids and peptide fragments...Ch. 29 - Prob. 29.59PCh. 29 - Prob. 29.60PCh. 29 - Prob. 29.61PCh. 29 - 29.59 An octapeptide contains the following amino...Ch. 29 - Prob. 29.63PCh. 29 - Prob. 29.64PCh. 29 - Draw all the steps in the synthesis of each...Ch. 29 - 29.62 Write out the steps for the synthesis of...Ch. 29 - 29.64 Another method to form a peptide bond...Ch. 29 - Prob. 29.68PCh. 29 - Prob. 29.69PCh. 29 - Which of the following amino acids are typically...Ch. 29 - After the peptide chain of collagen has been...Ch. 29 - Prob. 29.72PCh. 29 - Prob. 29.73PCh. 29 - 29.70 The anti-obesity drug orlistat works by...Ch. 29 - Prob. 29.75P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following compound is an intermediate in the biosynthesis of one of the 20 common -amino acids. Which one is it likely to be, and what kind of chemical change must take place to complete the biosynthesis?arrow_forwardWhich amino acids could be referred to as derivatives of butanoic acid?arrow_forwardwhich amino acids has a side chain that can serve as a hydrogen bond donor but not acceptor in its predominant form at pH7arrow_forward
- Draw the predominant form the arginine amino acids at physiological pH (7.4):arrow_forwardDraw all ionic structures for an amino acid under acidic and basic conditions, and identify the zwitterion.arrow_forwardVancomycin is made up of a peptide chain consisting of seven amino acids and there are 3 phenyl glycine,2 chlorinated tyrosine, aspartic acid, and N-methyl leucine. Locate and label itarrow_forward
- Write the complete structures for the following peptides. Tell whether each peptide is acidic, basic, or neutral.(a) methionylthreonine (b) threonylmethionine(c) arginylaspartyllysine (d) Glu-Cys-Glnarrow_forwardDraw out the peptide IYV and clearly label/identify all the Phi, Psi, and peptide bonds present in the peptide. Be sure to draw out the entire peptide and label the N- and C-termini. Which type of secondary structure would this peptide most likely take on (assuming it would take on a secondary structure)?.arrow_forwardUnder what condition will an amino acid have a pKa?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning
Biomolecules - Protein - Amino acids; Author: Tutorials Point (India) Ltd.;https://www.youtube.com/watch?v=ySNVPDHJ0ek;License: Standard YouTube License, CC-BY