
Concept explainers
(a)
Interpretation: Various products formed from the pyrolysis of butane with regard to only
Concept introduction: Long-chain petroleum hydrocarbons can be broken down unless highly elevated temperatures are used. At extremely elevated temperature the
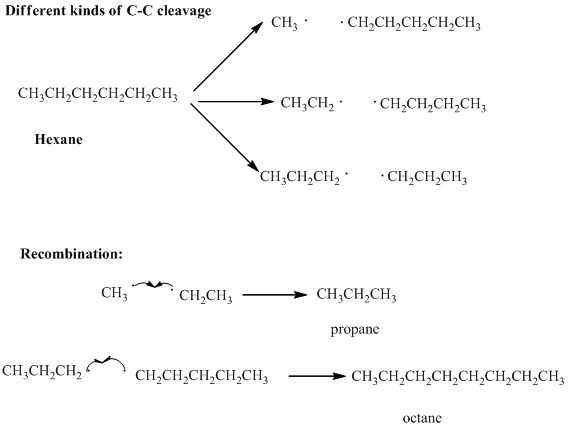
Thus the products have new covalent bonds formed as results of electrons contributed from different atoms.
(b)
Interpretation: Various products formed from the pyrolysis of
Concept introduction: : Long-chain petroleum hydrocarbons can be broken down unless highly elevated temperatures are used. At extremely elevated temperature the
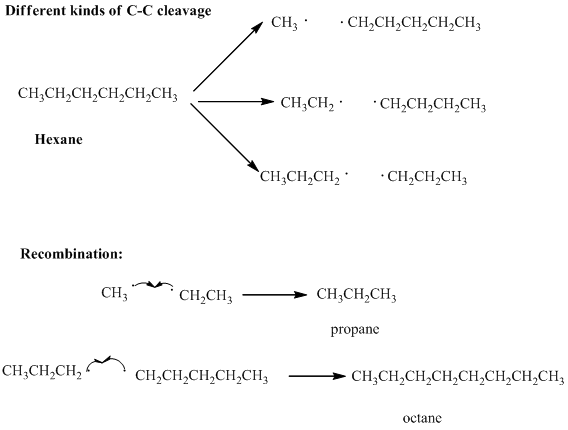
Thus the products have new covalent bonds formed as results of electrons contributed from different atoms.
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
ORGANIC CHEM F/UT (LOOSELEAF)
- 4-43 For each alkane, 1. draw all the possible monochlorinated derivatives. 2. determine whether free-radical chlorination would be a good way to make any of these monochlorinated derivatives. (Will the reaction give mostly one major product?) 3. which monobrominated derivatives could you form in good yield by free-radical bromination? (a) cyclopentane (c) 2-methylpentane (b) methylcyclopentane (d) 2,2,3,3-tetramethylbutanearrow_forwardThe rate of elimination of cis-1-bromo-4(1,1-dimethyl)cyclohexane is proportional to the concentration of both substrate and base, but that of the trans isomer is proportional only to the concentration of the substrate. a) draw the structures of cis and trans 1-bromo-4(1,1,-dimethyl)cyclohexane b) explain the difference in kinetics between the elimination reaction of cis and trans isomers.arrow_forwardDrawing on what you know about the stereochemistry of alkene addition reactions, write the mechanism for the reaction of 2-butyne with one equivalent of Br₂. Draw all missing reactants and/or products in the appropriate boxes by placing atoms on the canvas and connecting them with bonds. Add charges where needed. Electron flow arrows should start on an atom or a bond and should end on an atom, bond, or location where a new bond should be created. WW 10 [1] A Submit *H* EXP.¹ CONT. L Request Answer Marvin JS by ChemAxon H C N O S CI Br I P TI Farrow_forward
- 5. Consider the synthesis of 2-butanone from butyne: Hg2+ || CH3CH,-C=C–H CH;CH,-Ĉ-CH3 H3o* (iv) Name the class of compound D belongs to. (v) Comment on the stability of compound D compared to 3-butanone.arrow_forwardDraw structural formulas for the major organic product(s) of the reaction shown below. OCH3 H2SO4 HNO3 You do not have to consider stereochemistry. • If no reaction occurs, draw the organic starting material. Remember to include all of the formal charges on the atoms of any nitro groups. Draw one structure per sketcher. Add additional sketchers usıng the drop-down menu in the bottom rig Separate multiple products using the sign from the drop-down menu.arrow_forwardReaction of 2-methyl-2-butene (above) with HBr might, in principle, lead to a mixture of two alkyl bromide addition products. Draw these two alkyl bromides.arrow_forward
- Draw curved arrows and identify the thermal and photochemical products of the electrocyclic reaction of (2Z,4Z,6E)-octa-2,4,6-triene. Step 2: An electrocyclic reaction will undergo a concerted electron shift to form a cyclic ring. A new sigma bond will be formed between carbons 2 and 7. The product will be neutral. (2ZAZ,6E)-octa-2,4,6-triene Draw the curved arrows to show the mechanism. Select Draw Rings More Erasearrow_forward- For the dehydration shown, use curved arrows to show the formation of the carbocation intermediate in the presence of sulfuric acid H2SO4H2SO4, then draw the structures of the minor and major products of the elimination. Picture: Cyclohexane (no double or triple bonds) with a methyl group and an alcohol group (HO 4e- on O) on C1 (bottom) reacting with H2SO4 -> "Major and Minor Products" -The H2SO4H2SO4 is abbreviated as H+H+ in the drawing module. Do not delete any pre‑drawn bonds, charges, or lone pairs. If you accidentally make a mistake, remove the last change by using the undo button on the lower left or revert the drawing palette to the original state by selecting the More menu, then select Reset Drawing. Step 1: Use curved arrows to complete the protonation mechanism of the alcohol. Step 2: Use a curved arrow to show the formation of the carbocation intermediate for the elimination. (Picture 1) -Step 3: This tertiary carbocation intermediate readily undergoes…arrow_forward1. Predict the product(s) and propose a mechanism of the following nucleophilic substitution reaction. Remember that a mechanism should contain arrows to show movement of electrons, all intermediates and appropriate formal charges. Br + H₂O →arrow_forward
- Look at Figure 4-12 on page 105, and estimate the percentages of axial and equatorial conformations present at equilibrium in bromocyclohexane.arrow_forward6. Both acid-catalyzed hydration and oxymercuration-demercuration are addition reactions that add a hydroxyl group (--OH) and a hydrogen atom across a t-bond to make a Markovnikov product. Because of this, both reactions can often end with the same major organic product. 3 Explain in 1-3 complete sentences why substrate A leads to the same product no matter the conditions and why substrate B leads to two different products based on reaction conditions. 4 10 A ER A % H₂SO4 H₂O 1. Hg(OAc)2 2. NaBH4 當 5 OH Q Search T (racemic) OH (racemic) 6 & B B H₂SO4 H₂O 1. Hg(OAc)₂ 2. NaBH4 OH (racemic) lyi Oarrow_forwardOf the following halonium (bromonium) ions; Indicate for each one which of the two carbons is more susceptible to being attacked by the nucleophile and explain why. Br+ Br* Br Br X X CH3 CH3 H CH3 H |||4 H H3C... H 114 H Harrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

