
Concept explainers
Interpretation:
The curved arrows are to be added to the given reactions, which indicate the flow of electrons for all the bond-forming and bond-breaking steps.
Concept introduction:
Curved arrows are used to represent the direction of the flow of electrons in a reaction mechanism.
Curved arrows are drawn in such a way that they point from the source of an electron pair toward the atom, which receives the electron pair.
The direction of flow of electrons is always from a high electron density site to a low electron density site.
Curved arrows never represent the movement of atoms.
The movement of electrons shown by the curved arrows should not violate the octet rule for the elements, which are present in the second row of the periodic table.
Electrophiles are positive or partially positive species that attract electrons or negatively charged species toward itself.
Answer to Problem 1PP
Solution:
(a)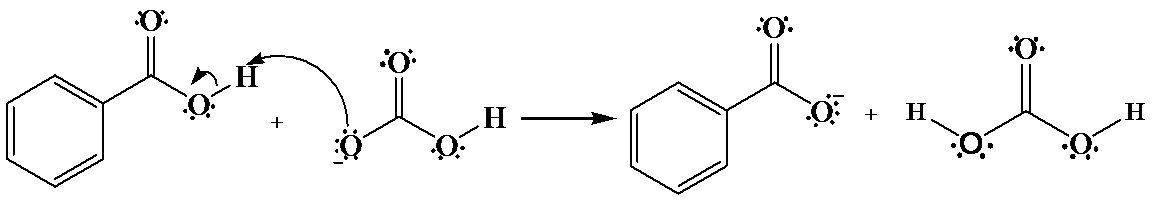
(b) 
Explanation of Solution
a)

The hydrogen of benzoic acid is partially positive and is electrophilic in nature. An oxygen atom provides an unshared pair of electrons, which forms bond with the benzoic acid hydrogen that cause the departure of a benzoate anion.
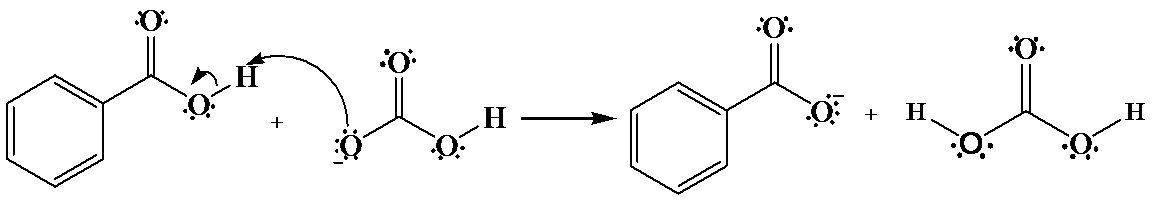
b)
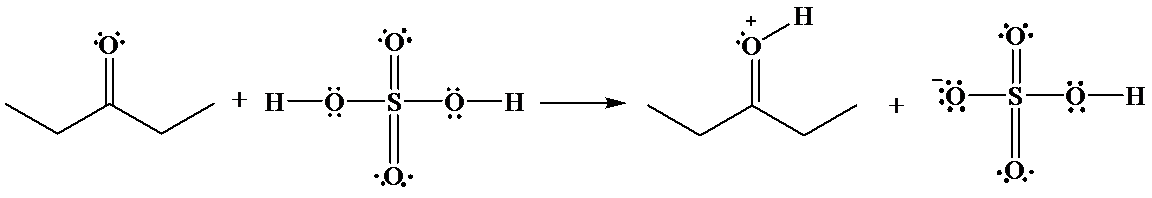
The hydrogen of sulfuric acid is partially positive and is electrophilic in nature. An oxygen atom of
The reaction is as follows:

Want to see more full solutions like this?
Chapter 3 Solutions
ORGANIC CHEM LL W/WILEYPLUS BLCKBRD >I
- Give a clear handwritten answer with explanation...give the mechanism with curved arrows and formal charges..arrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Long and detailed explanations are not needed. Keep it short, brief, and direct because I only need the answers. Which of the following statements is true for compounds A and B ?arrow_forwardProvide a detailed, stepwise mechanism for the following transformation. Use the curved arrow formalism to show the flow of electrons. Show all lone pairs, formal charges, etcarrow_forward
- Write a curved arrow mechanism for the following transformation:arrow_forwardWrite down the explicit structures of A, B, C and D in accordance with the reactions given in the synthesis plan, which shows the synthesis of D, a pheromone of moth.arrow_forwardProvide a detailed , stepwise mechanism for the following transformation . Use the curved arrow formalism to show the flow of electrons . Show all lone pairs , formal charges , and pertinent resonance structures .arrow_forward
- Which compound (i or ii) is the stronger base? Discuss your answer comprehensively by amoungst other providing an acid base reaction for one of the compounds.arrow_forwardPlease provide a detailed mechanism for the following transformation. Also, show the appropriate curved arrows to rationalize the next step. The quality of the electron pushing counts.arrow_forwardPlease answer this NEATLY, COMPLETELY, and CORRECTLY for an UPVOTE. Arrange the intermediates below in order of increasing basicity:arrow_forward
- Give detailed Solution with explanation needed....identify the structural formula that corresponding to the compound names. If you give correct answer all compounds I will give you upvotearrow_forwardGive a clear handwritten answer with explanation..Complete the following reactions?arrow_forwardFor the following groups of molecules (labeled A-C), rank the acidity of the molecules in order from least acidic (3) to most acidic (1). Explain your reasoning.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





