
To review:
The types of forces, which help the insulin to bind to its target, and the truth about the amino acids placed at positions B23, B24, A2, A19, and A3, which allow them to get involved in the substrate binding.
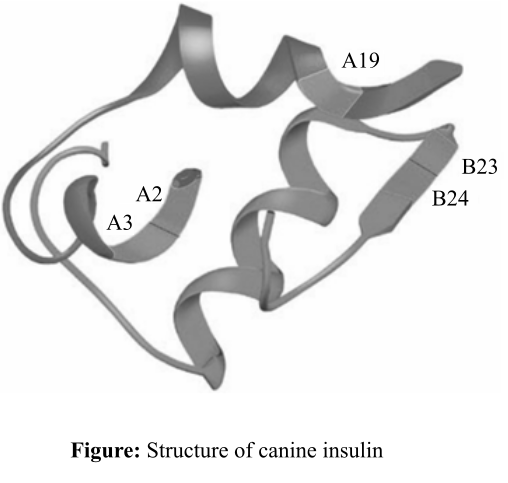
Figure: Structure of canine insulin.
Introduction:
The quaternary structure of the proteins involves two or more chains of the peptides that held together with the help of various covalent and noncovalent forces like hydrogen bonds, van der Waal forces of attraction, and disulfide linkages. An enzyme is held to its substrate with the help of van der Waal forces of attraction.
Explanation of Solution
Insulin is an enzyme, which helps to lower the blood glucose level by storing it in the form of glycogen in the muscle tissues. It binds to its substrate, that is, glucose by the noncovalent van der Waal interactions. The van der Waal interactions occur over a short distance and are weak forces. Generally, enzymes bind to their substrate with the help of these forces so that products formed could be detached easily from the enzyme and it again becomes available for the next cycle of reaction.
The part of the insulin enzyme, that is involved in van der Waal interaction, contains hydrophobic amino acid groups (−R group), for example, valine, isoleucine, glycine, and phenylalanine, which might have placed on the positions such as A2, A3, A19, B23, and B24. The –R groups protrude out of the insulin side chain so that they could interact with the target molecules or the amino acids that are present in the substrates.
Thus, it can be concluded that the noncovalent interactions like van der Waal forces help the insulin to bind to its substrates. Amino acids at the positions B23, B24, A2, A19, and A3 might contain hydrophobic side chain amino acid groups so that they help insulin to bind to substrates.
Want to see more full solutions like this?
- Essentials of Pharmacology for Health ProfessionsNursingISBN:9781305441620Author:WOODROWPublisher:Cengage
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning



