
Draw the structure of a compound fitting each description:
a. an
b. a
c. a carboxylic acid with molecular formula
d. an ester with molecular formula
(a)
Interpretation: The structure of an aldehyde with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
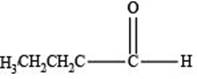
The structure of an aldehyde with molecular formula
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
Therefore, the given compound has one double bond with an aldehydic functional group. Hence, the structure an aldehyde with molecular formula
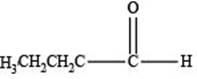
Figure 1
The structure of an aldehyde with molecular formula
(b)
Interpretation: The structure of a ketone with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
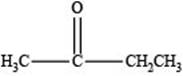
The structure of a ketone with molecular formula
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
Therefore, the given compound has one double bond with a ketone functional group. Hence, the structure a ketone with molecular formula

Figure 2
The structure of a ketone with molecular formula
(c)
Interpretation: The structure of a carboxylic acid with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
The structure of a carboxylic acid with molecular formula
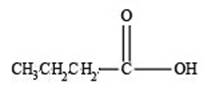
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
Therefore, the given compound has one double bond with a carboxylic acid functional group. Hence, the structure a carboxylic acid with molecular formula
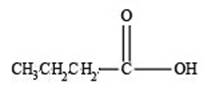
Figure 3
The structure of a carboxylic acid with molecular formula
(d)
Interpretation: The structure of an ester with molecular formula
Concept introduction: Functional groups are specific substituents present in the molecule that is responsible for the characteristic chemical reactions. For example, functional groups possess a carbonyl group includes aldehydes, ketones, carboxylic acids, amides, esters and acid chlorides. An organic compound containing
Answer to Problem 3.10P
The structure of an ester with molecular formula
Explanation of Solution
The given molecular formula is
Where,
• C is the number of carbon atoms.
• H is the number of hydrogen atoms.
Substitute the values of number of carbon atoms and hydrogen atoms in the above formula to calculate the number of double bond in compound
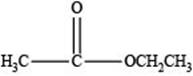
Therefore, the given compound has one double bond with an ester functional group. Hence, the structure an ester with molecular formula

Figure 4
The structure of an ester with molecular formula
Want to see more full solutions like this?
Chapter 3 Solutions
ORG.CHEMISTRY CONNECT ACCESS>CUSTOM<
- List the following compounds in order of increasing water solubility: a.ethoxyethane b.propanoic acid c.pentane d.1 butanolarrow_forwardDexamethasone is a halogen-containing steroid used to treat infl ammation in rheumatoid arthritis and other conditions. (a) Classify the alkyl halide in dexamethasone as 1 °, 2 °, or 3 °. (b) Classify the hydroxyl groups as 1 °, 2 °, or 3 °.arrow_forwarda. What is the chemical structure of biphenyl? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward
- a) what important physical property of alcohols differentiate them from the other functional groups? b) is the O-H bond strengthened or weakened by this effect? (broad band spectrums)arrow_forward1. Chief organic component of vinegar a. acetic acid b. formic acid c. benzoic acid d. butanoic acid 2. This term means without water. a. carbonyl b. hydroxyl c. anhydride d. carboyl 3. Compounds containing the cyano group. a. nitriles b. amides c. amines d. nitrates 4. General formula of a Grignard reagent. a. RCOX b. RCN c. RCOOH d. RMgX 5. Organic derivatives of ammonia, derived from replacing one, two or all three hydrogens of the ammonia. a. amide b. amine c. cyan d. nitro 6. Sulfur analogs of alcohols where the O in R-OH is replaced by sulfur. a. Thioesters b. Thiols c. Thioaldehydes d. Thioethers 7. General formula of alkanes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 8. General formula of alkenes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 9. General formula of alkynes. a. CnH2n b. CnH2n+2 c. CnH2n-2 d. R-OH 10. Which is soluble in water? a. methanol b. ethanol c. propanol d. all of the above 11. Which substance will have the highest boiling point? a. methanol b.…arrow_forward14. PART 3: Draw the structure for compound A.arrow_forward
- 6. Draw the correct structures for the following: a. what is the correct structure of 3-methyl 1-pentyne? b. what is the correct structure of butyl methyl amine? c. what is the correct structure of 3-methyl 1-pentyne? d. what is the correct structure of 2-methyl 2-butanol? e. what is the correct structure of pentanoic acid?arrow_forward"A research team synthesizes a novel organic compound 'X' with the molecular formula C5H8O2. When 'X' is treated with a deuterated acid (D2O), a single deuterium atom replaces a hydrogen atom, forming compound 'Y' (C5H7DO2). 'X' does not react with 2,4-Dinitrophenylhydrazine (2,4-DNP) but does react with both Tollens' reagent and Benedict's solution, forming a silver mirror and a red precipitate, respectively. Furthermore, 'X' undergoes catalytic hydrogenation over a palladium catalyst, consuming one mole of hydrogen to form a compound 'Z' (C5H10O2). Based on these observations, what is the most likely structure of compound 'X'?" A. Methyl vinyl ketone B. 3-Buten-2-one C. Acetoacetic ester D. 2-Hydroxypent-3-enalarrow_forward3. a. What is the chemical structure of benzoic acid, circle functional groups different than alkane,alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? __________________________arrow_forward
- What kind of solvent ingredients is usually used in the concentrations of 4-10 percent in skin care products and their function is to soften skin cells and to lessen wrinkles? A. Ethly acetate B. Alpha hydroxyl acids C. Phenols and phenol derivatives D. Aliphatic alcoholsarrow_forwardWhat structural characteristic is shared by the aldehydes and the ketones? A) They both are straight chain compounds. B) Aldehydes and ketones both contain a carbonyl carbon. C) Both of these compound classes have as the smallest compound a 5 carbon skeleton. D) Aldehydes and ketones have no shared characteristics.arrow_forwardDraw the structure of a molecule that fi ts each description: a. a 2 ° alcohol of molecular formula C 6H 12O b. a cyclic ether with molecular formula C 5H 10O c. a 1 ° alkyl halide with molecular formula C 5H 11Clarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





