
Concept explainers
(a)
Interpretation:
The structure for the product of each of the given Lewis acid-base association reactions is to be derived by using curved-arrow notation. The formal charge on each species is to be assigned. The Lewis acid and the Lewis base is to be labeled. The atom that donates electrons in each case is to be predicted.
Concept introduction:
Lewis acid-base association reaction involves the transfer of a proton from a base to an acid. Lewis acid accepts an electron pair and acts as an electrophile. Lewis base donates an electron pair and acts as a nucleophile.
Answer to Problem 3.1P
The Lewis acid and Lewis base are
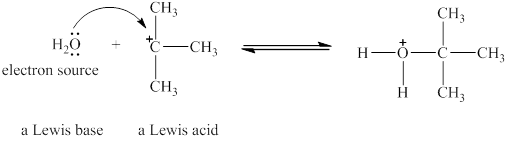
Explanation of Solution
Lewis acids are electron donor, whereas Lewis bases are electron pair accepter. In water molecule oxygen consists of a lone pair. Thus, it will act a Lewis base and oxygen atom has tendnecy to donate its electrons. The
The oxygen in
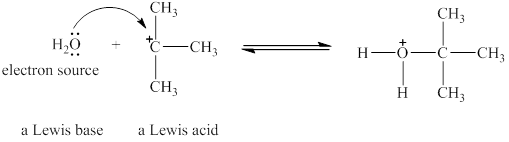
Figure 1
The Lewis acid and Lewis base are
(b)
Interpretation:
The structure for the product of each of the given Lewis acid-base association reactions is to be derived by using curved-arrow notation. The formal charge on each species is to be assigned. The Lewis acid and the Lewis base is to be labeled. The atom that donates electrons in each case is to be predicted.
Concept introduction:
Lewis acid-base association reaction involves the transfer of a proton from a base to an acid. Lewis acid accepts an electron pair. It acts as an electrophile Lewis base donates an electron pair. It acts as nucleophile.
Answer to Problem 3.1P
The Lewis acid and Lewis base are
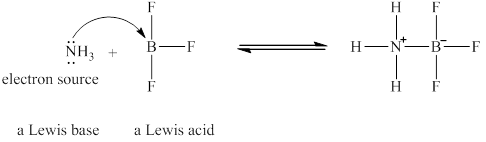
Explanation of Solution
Lewis acids are electron donor, whereas Lewis bases are electron accepter. In
The nitrogen in
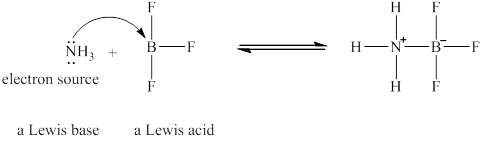
Figure 2
The Lewis acid and Lewis base are
Want to see more full solutions like this?
Chapter 3 Solutions
Organic Chemistry 6e & Study Guide
- Give a clear handwritten answer with explanation..given below a reaction label the acid ,conjugate acid, base ,andconjugate base..and tell the side of equilibrium is favored then show the electron pushing arrows to reflect the mechanism of actionarrow_forwardGive detailed Solution..give a reaction for weak acidarrow_forwardat a pH value of one, what will be the structures observed for both a and b?arrow_forward
- Acid-base behaviour in non aqueous solvents. Discuss with illustrationsarrow_forwardDraw the structure of the conjugate base of the acid given below. (Note that the acidic H in consideration is bold and underlined.)arrow_forwardProvide detailed (arrow pushing) mechanism for the following reaction, draw all importantresonance structures for the intermediates. Where appropriate indicate Lewis acid and base foreach step, and whether they are also Bronsted acids and bases (LB/BA, LA/BA etc.) Indicate thenumber of steps in the mechanism and the number of intermediates.arrow_forward
- Order the following in increase in basicity property and give reason for order.arrow_forwardDirections: A list of organic compounds is given on the 1st column. Check the boxes of qualitative test of which you think the compound will give a POSITIVE RESULT.arrow_forwardGive a clear handwritten answer with explanation,,??arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

