
Identify the
amide, and
a. 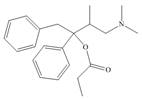 c.
c. 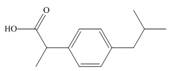 e.
e. 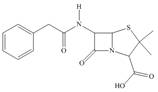
Darvon ibuprofen penicillin G
(analgesic) (analgesic) (an antibiotic)
b. 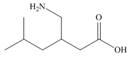 d.
d. 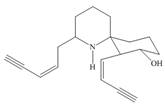 f.
f. 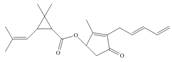
pregabalin histrionicotoxin pyrethrin I
trade name Lyrica (poison secreted by a (potent insecticide
(used in treating chronic south American frog) from chrysanthemum)
Pain)
(a)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.32P
The functional groups present in Darvon are shown below.
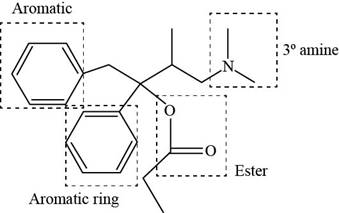
Explanation of Solution
The given compound is,
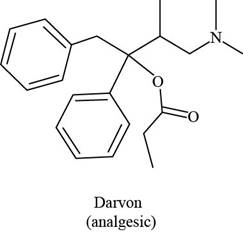
Figure 1
The functional groups present in Darvon are amine, ester and aryl group.
The structure of Darvon that shows the labeling of all functional groups is shown below.

Figure 2
The functional groups present in Darvon and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(b)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.32P
The functional groups present in pregabalin are shown below.
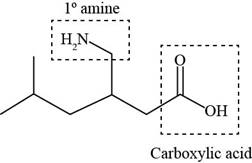
Explanation of Solution
The given structure is,
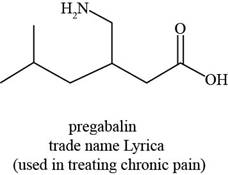
Figure 3
The functional groups present in pregabalin are amine, and carboxylic acid.
The structure of pregabalin that shows the classification of all functional group is shown below.

Figure 4
The functional groups present in pregabalin and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(c)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.32P
The functional groups present in ibuprofen are shown below.
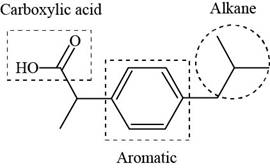
Explanation of Solution
The given structure is,
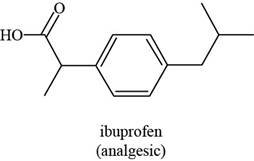
Figure 5
The functional groups present in ibuprofen are carboxylic acid, alkane and aryl.
The structure of ibuprofen that shows the classification of all functional group is shown below.

Figure 6
The functional groups present in ibuprofen and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(d)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.32P
The functional groups present in histrionicotoxin are shown below.
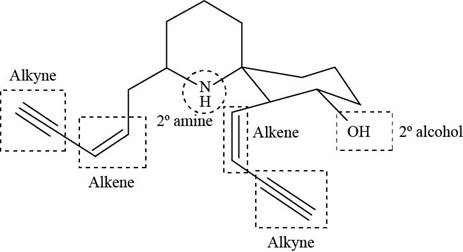
Explanation of Solution
The given structure is,
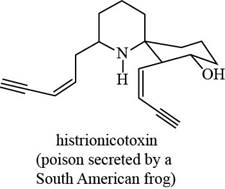
Figure 7
The functional groups present in histrionicotoxin are alkene, alkyne and alcohol. The structure of histrionicotoxin that shows the labeling of all functional group is shown below.

Figure 8
The functional groups present in histrionicotoxin and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(e)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.32P
The functional groups present in penicillin G are shown below.
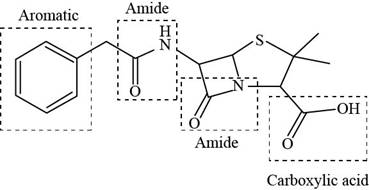
Explanation of Solution
The given structure is,
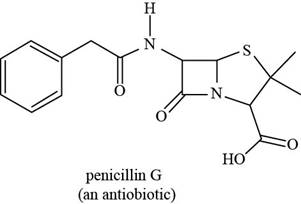
Figure 9
The functional groups present in penicillin G are carboxylic acid, amide, and aryl.
The structure of penicillin G that shows the labeling of all functional group is shown below.

Figure 10
The functional groups present in penicillin G and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
(f)
Interpretation: The functional groups present in the given molecule is to be classified and the classification of each alcohol, alkyl halide, amide, and amine as
Concept introduction: The table representing the general formula of different functional groups according to IUPAC convention is given below.
| General formula | Functional group | Functional group name |
| Alcohol | ||
| Aldehyde | ||
| Ketone | ||
| Amine | ||
| Ether | ||
| Ester | ||
| Alkyl halide | ||
| Double bond | ||
| Carboxylic acid |
The classification of alcohols or alkyl halides depend upon the number of carbon atoms attached to the carbon that contains hydroxyl group or alkyl halide. In case of primary alcohol,
The classification of amines or amides also depends upon the number of carbon atoms attached to the carbon that contains nitrogen atom.
Answer to Problem 3.32P
The functional groups present in pyrethrin I are shown below.
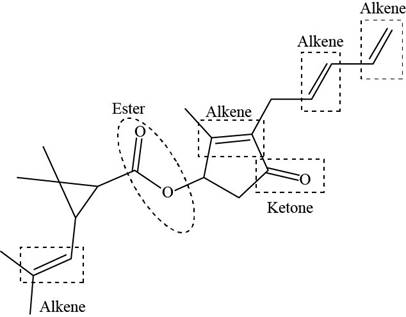
Explanation of Solution
The given structure is,
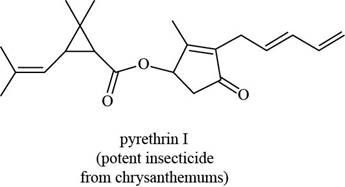
Figure 11
The functional groups present in pyrethrin I are ester, ketone, and alkene.
The structure of pyrethrin I that shows the labeling of all functional group is shown below.

Figure 12
The functional groups present in pyrethrin I and the classification of each alcohol, amide, alkyl halide and amine as primary, secondary, and tertiary are rightfully stated.
Want to see more full solutions like this?
Chapter 3 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
- No explanantion or anything needed just draw the compounds in organic chemarrow_forward(a) Identify the functional groups in salinosporamide A, an anticanceragent isolated from marine sediment. (b) Classify each alcohol, alkylhalide, amide, and amine as 1°, 2°, or 3°.arrow_forwardComplete the tables by providing the names of the functional groups corresponding to each letter. For any alcohol, alkyl halide, amide, or amine in the moleculeclassify it as 1, 2, or 3°Consider the functional groups in histrionicotoxi Classification: A. 1 B. 2 C. 3 D. Not applicablearrow_forward
- Please help by identifying each functional group. There is at least 2 steps for eacharrow_forwardExplain why a 1 ° amine and a 3 ° amine having the same number of carbons are soluble in water to a similar extent, but the 1 ° amine has a higher boiling point.arrow_forwardWhat amine is formed by reduction of each amide?arrow_forward
- HCl (aq), Zn(Hg) Br2, FeBr3 NBS, light KMnO4, H3O+ Mg metal, ether KOH, EtOH, heat CH3Cl, AlCl3 Dilute H3O+ ClCO(CH2)2CH3, AlCl3 NaCCCH2CH3 HNO3, H2SO4 2-butanonearrow_forwardWhy is ethylamine soluble in water while aniline is not? Explain in detail (4 sentences minimum).arrow_forwardConsider compound I below, which is structurally related to a natural product that was isolated from an extract of a Caribbean marine sponge (See J. Chem. Soc. 1994, 116, 6015). Answer the following questions about this compound ( Please Explain Answers) How primary alcohols are present? _________ How many secondary alcohols are present? _________ How many quaternary carbons are present? _______arrow_forward
- a. What is the chemical structure of 2,6-dichloroindophenol, circle functional groups differentthan alkane, alkene, alkyne? b. Is it polar or nonpolar? _______________________ c. What is its water solubility in g/L? ___________arrow_forwardi. Why is the boiling point of the aldehyde greater than that of the alkane? ii. Why is the boiling point of alcohol the highest? iii. Explain why the solubility of aldehydes and alcohols falls as the molecules get bigger.arrow_forwardWhat are the functional groups present in this image? A. Amide, carbonyl, and nitro B. Alkene, amine, and amide C. Amine and carbonyl D. Amine and amide only A brief explanation would be highly appreciated + upvotearrow_forward
 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning


