
Concept explainers
(a)
Interpretation:
The hybridization of each nonhydrogen atom in norethynodrel is to be determined.
Concept introduction:
Answer to Problem 3.38P
| Atom | Hybridization |
| sp |
Explanation of Solution
The structure of Norethynodrel is as below:
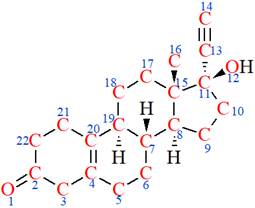
The nonhydrogen atoms are highlighted with red color and numbered.
According to VSEPR theory,
According to VSEPR theory,
According to VSEPR theory, the
According to VSEPR theory,
According to VSEPR theory, geometry of an atom has been determined, and from the geometry of the atom, its hybridization has been determined.
(b)
Interpretation:
The total number of
Concept introduction:
Answer to Problem 3.38P
There are total fifty-one
Explanation of Solution
The structure of Norethynodrel is as below:
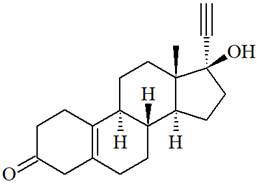
There is one C=C triple bond in norethynodrel molecule. Each triple bond is composed of two
From the
Want to see more full solutions like this?
Chapter 3 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





