
Concept explainers
a)
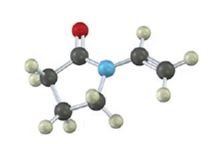
Interpretation:
Draw the structures of the
Concept introduction:
The term “polymer” is derived from the two words poly+mer. Where poly means ‘many’ and mer means ‘unit’, when combined known as polymer. The
The reaction mechanism may be classified into two categories:
Chain growth polymerization:
Chain growth polymers are produced by chain growth polymerization or addition polymerization. In this process an initiator is added to the reaction mixture. This initiator gets added to the carbon-carbon double bond and yields a reactive monomer (intermediate). This reactive intermediate reacts with the monomer and this process keeps on repeating to give rise to final polymeric product.
Step growth polymerization:
Step growth polymers are produced by step growth polymerization or condensation polymerization. In this process, each bond is formed in a step wise manner. In this the product of each is again a bi-functional group and the condensation goes on.
b)
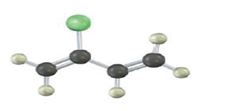
Interpretation:
Draw the structures of the polymers formed by the given monomer units.
Concept introduction:
The term “polymer” is derived from the two words poly+mer. Where poly means ‘many’ and mer means ‘unit’, when combined known as polymer. The polymerization is the process of reacting monomer molecules together in a chemical reaction to give rise to a polymer. If the reacting units are same then this type of polymer is known as homopolymer and if the reaction takes place between different molecules then it is called copolymer.
The reaction mechanism may be classified into two categories:
Chain growth polymerization:
Chain growth polymers are produced by chain growth polymerization or addition polymerization. In this process an initiator is added to the reaction mixture. This initiator gets added to the carbon-carbon double bond and yields a reactive monomer (intermediate). This reactive intermediate reacts with the monomer and this process keeps on repeating to give rise to final polymeric product.
Step growth polymerization:
Step growth polymers are produced by step growth polymerization or condensation polymerization. In this process, each bond is formed in a step wise manner. In this the product of each is again a bi-functional group and the condensation goes on.
Want to see the full answer?
Check out a sample textbook solution
Chapter 31 Solutions
Organic Chemistry
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





