
Interpretation:
To state that the stereoisomer of polypropylene will rotate the plane polarized light or not.
Concept introduction:
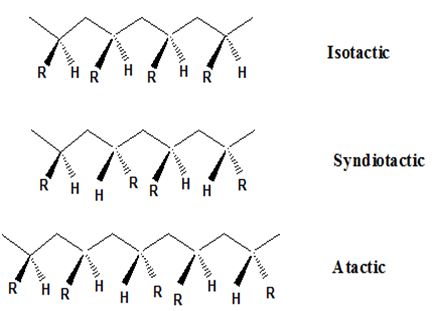
Isotactic, syndiotactic, and atactic are the stereochemical forms. The
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta percha is also an example for Syndiotactic polymer.
In atactic form the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain growth
If the mixtures of the stereoisomers obtained are racemic in nature then they will not rotate the plane polarized light.
The racemic mixture or racemate is defined as the one that has equal amounts of left and right handed enantiomers of the chiral carbon.
Trending nowThis is a popular solution!

Chapter 31 Solutions
Organic Chemistry
- What is molecular shape/conformation of polymer? What is the main characteristic ofpolymer affected by molecular shape?arrow_forwardDiscuss chirality and isomerization and their importance for understanding biodegradation.arrow_forward5. 1) Discuss how polymerization changes the properties of any ensemble of amino acids so that a protein polymer functions essentially differently than an aggregate of amino acids. 2) Why does a protein need to be a polymer in order to properly function??arrow_forward
- "Co-polymers" consist of two different monomers ("A" and "B") joined in analternating fashion (ABABAB...). Block co-polymers also consist of twodifferent monomers, but in this case, blocks of polymer containing only Aunits are joined to blocks of polymer containing only B units(AAAAABBBBB...). How block co-polymers are synthesized?arrow_forwardPeter Parker is looking for new materials for his synthetic web. Materials generally involve polymers, which are long, repeating molecules, which can be linear or branched, which can have molecular masses in the order of thousands or tens of thousands of Daltons. To prepare polymers, advanced reactions are usually required, such as coupling reactions, Ziegler – Natta, advanced oxidations, among others. The characteristics of the polymer depend of the properties of the geometry and polarity of its precursors, so Parker decides to study different precursors proposed by Tony Stark. Help Parker with his investigation: a) Stark proposes to Parker that he can use some anions as crosslinking ligands between cationic polymers to increase the strength of your webs. He proposes you to try the anions of sodium dithionite (Na2S2O4), lithium borohydride (LiBH4), calcium hexafluorophosphate (Ca (PF6)2), ammonium fluorophosphate ((NH4)2PO3F) and sodium thiosulfate (Na2S2O3). Propose the most probable…arrow_forwardIf a molecule contains stereocentres, is it guaranteed to be chiral?arrow_forward
- What type of stereochemistry, S or R, does the chiral center in this molecule have?arrow_forwardWhat are the requirements on the four groups attached to a carbon atom in orderthat it be a chiral center?arrow_forwardGive handwritten answer- Write three characteristic features for Zieglar Natta polymerization.arrow_forward
- the monomer units of amylose, the main component of starch, andglycogen, the animal stored carbohdrate, are both composed of a a-D-glucose monomers. however, the structural differences between these two polymers are remarkable: amylose is a coiled single stranded polysaccharaide while glycogen forms long branched networks. Explain the difference.arrow_forwardIn copolymers made of ethylene and vinyl acetate monomers, melting point anddegree of crystallinity decrease as the percentage of vinyl acetate increases.Suggest an explanation.arrow_forwardHow are polymers classified on the basis of mode of polymerization? Explain with suitable examples.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning





