
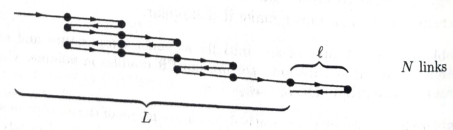
(a) Find an expression for the entropy of this system in term of N and
(b) Write down a formula for L in terms of N and N1,
(c) For a one-dimensional system such as this, the length L is analogous to the volume V of a three-dimensional system. Similarly, the pressure P is replaced by the tension force F. Taking F to be positive when the rubber band is pulling inward, write down and explain the appropriate
(d) Using the thermodynamic Identity, you can flow express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T, N, and
(e) Show that when
(f) Discuss the dependence of the tension force on temperature. If you increase the temperature of a rubber band, does it tend to expand or contract? Does this behavior make sense?
(g) Suppose that you hold a relaxed rubber band in both hands and suddenly stretch it. Would you expect its temperature to increase or decrease? Explain. Test your prediction with a real rubber band (preferably a fairly heavy one with lots of stretch), using your lips or forehead as a thermometer. (Hint: The entropy you computed in part (a) is not the total entropy of the rubber band. There is additional entropy a8sociated with the vibrational energy of the molecules; this entropy depends on U but is approximately independent of L.)
Want to see the full answer?
Check out a sample textbook solution
Chapter 3 Solutions
An Introduction to Thermal Physics
Additional Science Textbook Solutions
Glencoe Physical Science 2012 Student Edition (Glencoe Science) (McGraw-Hill Education)
Life in the Universe (4th Edition)
Conceptual Integrated Science
Physics: Principles with Applications
Conceptual Physics (12th Edition)
Applied Physics (11th Edition)
- Polymers, like rubber, are made of very long molecules, usually tangled up in a configuration that has lots of entropy. As a very crude model of a rubber band, consider a chain of N links, each of length L Imagine that each link has only two possible states, pointing either left or right. The total length L of the rubber band is the net displacement from the beginning of the first link to the end of the last link. Using the thermodynamic identity, you can now express the tension force F in terms of a partial derivative of the entropy. From this expression, compute the tension in terms of L, T , N, and l.arrow_forwardUsing the information provided (image), compare the change in entropy during the isothermal expansion from 1 L to 2 L of one mole of a rigid diatomic ideal gas and one mole of a rigid diatomic Van der Waals gas with b = 0.08 L/mol (ethanol). Are the changes in entropy the same for both or is one greater than the other?arrow_forwardIn this problem you are to consider an adiabaticexpansion of an ideal diatomic gas, which means that the gas expands with no addition or subtraction of heat. Assume that the gas is initially at pressure p0, volume V0, and temperature T0. In addition, assume that the temperature of the gas is such that you can neglect vibrational degrees of freedom. Thus, the ratio of heat capacities is γ=Cp/CV=7/5. Note that, unless explicitly stated, the variable γshould not appear in your answers--if needed use the fact that γ=7/5 for an ideal diatomic gas. Find an analytic expression for p(V), the pressure as a function of volume, during the adiabatic expansion. Express the pressure in terms of V and any or all of the given initial values p0, T0, and V0. p(V) = __________arrow_forward
- In this problem you are to consider an adiabaticexpansion of an ideal diatomic gas, which means that the gas expands with no addition or subtraction of heat. Assume that the gas is initially at pressure p0, volume V0, and temperature T0. In addition, assume that the temperature of the gas is such that you can neglect vibrational degrees of freedom. Thus, the ratio of heat capacities is γ=Cp/CV=7/5. Note that, unless explicitly stated, the variable γshould not appear in your answers--if needed use the fact that γ=7/5 for an ideal diatomic gas. A) Find an analytic expression for p(V), the pressure as a function of volume, during the adiabatic expansion. Express the pressure in terms of V and any or all of the given initial values p0, T0, and V0. p(V) = __________ B) At the end of the adiabatic expansion, the gas fills a new volume V1, where V1>V0. Find W, the work done by the gas on the container during the expansion. Express the work in terms of p0, V0, and V1. Your…arrow_forwardWhen an aluminum bar is temporarily connected between a hot reservoir at 725 K and a cold reservoir at 310 K, 2.50 kJ of energy is transferred by heat from the hot reservoir to the cold reservoir. In this irreversible process, calculate the change in entropy of (a) the hot reservoir, (b) the cold reservoir, and (c) the Universe, neglecting any change in entropy of the aluminum rod. (d) Mathematically, why did the result for the Universe in part (c) have to be positive?arrow_forwardConsider a discrete random variable X with 2n+1 symbols xi, i = 1, 2, …, 2n+1. Determine the upper and lower bounds on the entropy when (a) p(x1)=0 (b) p(x1)=1/2arrow_forward
- Hi, could I get some help with this macro-connection physics problem involving isothermal expansion? The set up is: For an isothermal reversible expansion of two moles of an ideal gas, what is the entropy change of the a) gas and b) the surroundings in J/K to 4 digits of precision if the gas volume quadruples, assuming NA = 6.022e23 and kB = 1.38e-23 J/K? Thank you.arrow_forwardplease do fast 5. Find the expression for the entropy of a single harmonic oscillator.arrow_forwardThe volume of a monatomic ideal gas triples in an isothermalexpansion. By what factor does its pressure change?arrow_forward
- Can you show that ∂U/∂P )T =0 J/Pa for a perfect gas? Hint: Start with ∂U = T ∂S − P ∂V. Quickly, you will come to a derivative of entropy; to get rid of it to answer the question, use a Maxwell relation.arrow_forwardImagine a photon gas at an initial temperature of T = 1.4 K. What is the temperature of the photon gas (in K) after it has undergone a reversible adiabatic expansion to 2 times its original volume?arrow_forwardIn Debye Approximation the entropy at some temperature T (less than 10 K) is aT(Blank 1 ) /3 If the value of this entropy at T = 3.5 K is 1.55 J / K , then the value of the coefficient "a" is : ( Blank 2)arrow_forward
 Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
Modern PhysicsPhysicsISBN:9781111794378Author:Raymond A. Serway, Clement J. Moses, Curt A. MoyerPublisher:Cengage Learning
