
An Introduction to Thermal Physics
1st Edition
ISBN: 9780201380279
Author: Daniel V. Schroeder
Publisher: Addison Wesley
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 3.1, Problem 3P
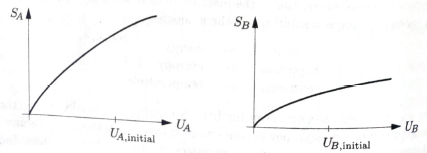
Figure 3.3 shows graphs of entropy vs. energy for two objects, A and B. Both graphs are on the same scale. The energies of these two objects initially have the values indicated; the objects are then brought into thermal Contact with each other. Explain what happens subsequently and why, without using the word “temperature.”
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A refrigerator has a COP of 1.5. That is, the refrigerator removes 1.5 kWh of energy from the refrigerated space for each 1 kWh of electricity it consumes. Is this a violation of the first law of thermodynamics? Explain.
The first low of the thermodynamics shows U = Q - W, where U is a changing internal energy of the gas in the system, Q is a heat that the system received, and DW is work done by the system. If 15 kJ of heat flows out of a system and 30 kJ of work is done by the system, then what is the change in internal energy?
A typical temperature for surface water in a tropical ocean is 27°C. Whereas at a depth of a kilometer or more it is only about 27°C whereas at a depth of a kilometer or more it is only about 5°C. It has been proposed to operate heat engines using surface water as the hot reservoir and deep water as the cold reservoir. What would the maximum efficiency of such an engine be? Why might such engine eventually be practical proposition even with so low an efficiency?
Chapter 3 Solutions
An Introduction to Thermal Physics
Ch. 3.1 - Use Table 3.1 to compute the temperature of solid...Ch. 3.1 - Use the definition of temperature to prove the...Ch. 3.1 - Figure 3.3 shows graphs of entropy vs. energy for...Ch. 3.1 - Can a miserly system, with a concave-up...Ch. 3.1 - Prob. 5PCh. 3.1 - Prob. 6PCh. 3.1 - Prob. 7PCh. 3.2 - Prob. 8PCh. 3.2 - In solid carbon monoxide, each CO molecule has two...Ch. 3.2 - An ice cube (mass 30 g) at 0C is left sitting on...
Ch. 3.2 - In order to take a nice warm bath, you mix 50...Ch. 3.2 - Estimate the change in the entropy of the universe...Ch. 3.2 - When the sun is high in the sky, it delivers...Ch. 3.2 - Experimental measurements of the heat capacity of...Ch. 3.2 - Prob. 15PCh. 3.2 - A bit of computer memory is some physical object...Ch. 3.3 - Prob. 17PCh. 3.3 - Prob. 18PCh. 3.3 - Prob. 19PCh. 3.3 - Prob. 20PCh. 3.3 - Prob. 21PCh. 3.3 - Prob. 22PCh. 3.3 - Prob. 23PCh. 3.3 - Prob. 24PCh. 3.3 - Prob. 25PCh. 3.3 - Prob. 26PCh. 3.4 - What partial-derivative relation can you derive...Ch. 3.4 - A liter of air, initially at room temperature and...Ch. 3.4 - Sketch a qualitatively accurate graph of the...Ch. 3.4 - As shown in Figure 1.14, the heat capacity of...Ch. 3.4 - Experimental measurements of heat capacities are...Ch. 3.4 - A cylinder contains one liter of air at room...Ch. 3.4 - Prob. 33PCh. 3.4 - Polymers, like rubber, are made of very long...Ch. 3.5 - Prob. 35PCh. 3.5 - Prob. 36PCh. 3.5 - Prob. 37PCh. 3.5 - Suppose you have a mixture of gases (such as air,...Ch. 3.6 - Prob. 39P
Additional Science Textbook Solutions
Find more solutions based on key concepts
17. A speed skater moving to the left across frictionless ice at 8.0 m/s hits a 5.0-m-wide patch of rough ice....
Physics for Scientists and Engineers: A Strategic Approach with Modern Physics (4th Edition)
45. Suppose where vector has components Ax = 5, Ay = 2 and vector B has components Bx = –3, By = –5.
a. What ...
College Physics: A Strategic Approach (4th Edition)
Explain all answers clearly, with complete sentences and proper essay structure if needed. An asterisk(*) desig...
Cosmic Perspective Fundamentals
9. When steam changes phase to water, it
absorbs energy.
releases energy.
neither absorbs nor releases energy.
...
Conceptual Physical Science (6th Edition)
The circuit at tight contains three identical bulbs and an ideal battery. Assume that the resistance of the swi...
Tutorials in Introductory Physics
3. The back wall of an auditorium is 26.0 m from the stage. If you are seated in the middle row, how much time ...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- A copper rod of cross-sectional area 5.0 cm2 and length 5.0 m conducts heat from a heat reservoir at 373 K to one at 273 K. What is the time rate of change of the universe's entropy for this process?arrow_forwardCalculate the increase in entropy of the Universe when you add 20.0 g of 5.00C cream to 200 g of 60.0C coffee. Assume that the specific heats of cream and coffee are both 4.20J/g C.arrow_forwardConsider the thermodynamic process, A->B->C->A shown above. The heat absorbed during A->B is 591J. If the change in internal energy during B->C is 4146J, What is the change in internal energy in SI units during C->A? Express only the number of your answer with 4 significant figures.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
 Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Principles of Physics: A Calculus-Based TextPhysicsISBN:9781133104261Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and Engineers, Technology ...PhysicsISBN:9781305116399Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning
An Introduction to Physical SciencePhysicsISBN:9781305079137Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar TorresPublisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning


Principles of Physics: A Calculus-Based Text
Physics
ISBN:9781133104261
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Physics for Scientists and Engineers, Technology ...
Physics
ISBN:9781305116399
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

An Introduction to Physical Science
Physics
ISBN:9781305079137
Author:James Shipman, Jerry D. Wilson, Charles A. Higgins, Omar Torres
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY