
Concept explainers
(a)
Interpretation:
The molecule given is polar or not has to be predicted, if it is polar by using a vector the direction in the electrons are pulled has to be indicated.
(a)
Explanation of Solution
The given molecule is
The geometry of the molecule has to be determined first. To determine the polarity, draw the Lewis structure.
Distribute valence electron:
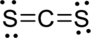
Vectors can be used to determine the polarity of this compound. The vectors should point in the direction of each electronegative sulfur atom. Since the dipole moment cancels each other, the molecule is not polar.
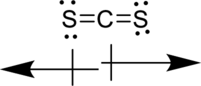
(b)
Interpretation:
The molecule
(b)
Explanation of Solution
The given molecule is
The geometry of the molecule has to be determined first. To determine the polarity, draw the Lewis structure.
Distribute valence electron:
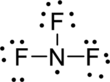
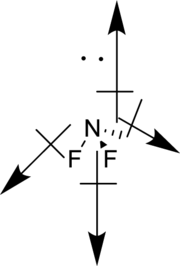
Vectors can be used to determine the polarity of this compound. The vectors should point in the direction of electronegative fluorine atom. The given compound is polar.
(c)
Interpretation:
The molecule given is polar or not has to be predicted, if it is polar by using a vector the direction in the electrons are pulled has to be indicated.
(c)
Explanation of Solution
The given molecule is
The geometry of the molecule has to be determined first. To determine the polarity, draw the Lewis structure.
Distribute valence electron:
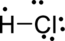
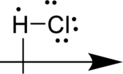
The given molecule is polar because there is an electronegativity difference between two atoms.
(d)
Interpretation:
The molecule given is polar or not has to be predicted, if it is polar by using a vector the direction in the electrons are pulled has to be indicated.
(d)
Explanation of Solution
The given molecule is
The geometry of the molecule has to be determined first. To determine the polarity, draw the Lewis structure.
Distribute valence electron:
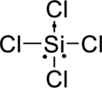
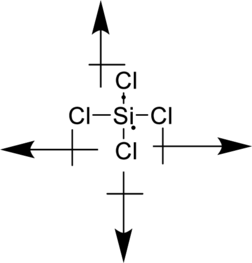
Vectors can be used to determine the polarity of this compound. The vectors should point in the direction of each electronegative chlorine atom. Since the dipole moment cancels each other, the molecule is non-polar.
Want to see more full solutions like this?
Chapter 3 Solutions
Connect 2-Year Online Access for General, Organic, and Biochemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





