
Concept explainers
Interpretation:
The labeled bonds in the given compound are to be arranged in the order of increasing bond lengths.
Concept introduction:
In hybridization, one
Answer to Problem 4.1P
The increasing order of bond-lengths in the given compound is
Explanation of Solution
The given compound is,
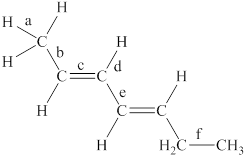
Figure 1
The bond with higher percentage of s-character has electron density closer to the nucleus and thus, has shorter bond length. The order of percentage
• Bond ‘a’ is present between the
• Bond ‘c’ is present between the two
• Due to the conjugation between two pi bonds, the bond length of ‘e’ is in between the carbon-carbon single bond and carbon-carbon double bond.
• Bond ‘b’ is present between one
• The
From the above points, it is concluded that the order of bond-lengths in the given compound is
The increasing order of bond-lengths in the given compound is
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Select the bond that must break when these electrons flow as shown by the mechanistic arrowarrow_forwardplease explain in detail give reason for each pairarrow_forwardGive detailed Solution with explanation needed..don't give Handwritten answer....which of the most stable chair form of the given compound?arrow_forward
- Please draw a step by step arrow pushing mechanism. Make it very detailed and include all arrows and include ALL resonance structures.arrow_forwardKindly explain why COSe is the answer in detail.arrow_forwardCan you explain how an alkene bonds to a metal like in this compound using some orbital diagrams?arrow_forward
- Draw each hybridisation steps (with detailed explanation) of this reaction pleasearrow_forwardDraw the resonance structure of the ff. and identify if aromatic or nonaromatic through Huckel’s rule. note: I need the answer immediately. I will send a good rate right away as well.arrow_forwardKindly answer this question a, b ,carrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning

