
Concept explainers
What is the geometry around the central atom in the following molecular models? (There are no “hidden” atoms: all atoms in each model are visible.)
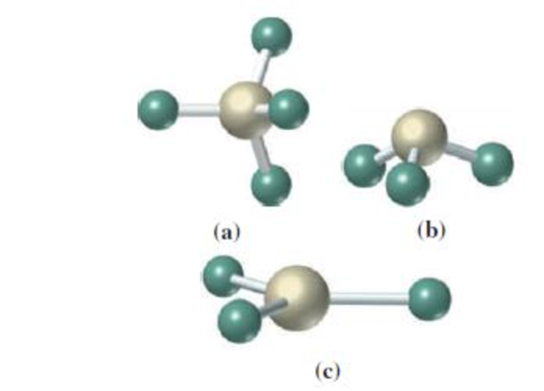
(a)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like a trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is tetrahedral.
Explanation of Solution
In the given molecular model,
Four atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is tetrahedral.
(b)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like a trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is pyramidal.
Explanation of Solution
In the given molecular model,
Three atoms are bonded to the central atom. Three atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is pyramidal.
(c)
Interpretation:
The geometry around the central atom in the given molecular model is to be determined.
Concept introduction:
Molecular shape can be predicted from the Lewis structure by using the valence-shell Electron-pair repulsion (VSEPR) model.
- Count the number of valence electron pairs (bond pairs and lone pairs).
- Assume that the valence electron pairs form a structure that allows them to be as far away from each other as possible.
- If there are only two bond pair electrons, the molecule is linear.
- If there are three bond pair electrons, the molecule is shaped like trigonal planar.
- If there are four bond pair electrons, the molecule is shaped as a regular tetrahedral.
- Repulsion between lone pair-bond pair of electrons effect the geometry of molecules.
Bond angle is the angle between two bonds of a molecule and it is determined based on the geometry.
[Bond angles:
Answer to Problem 4.25UKC
The geometry around the central atom in the given molecular model is trigonal planar.
Explanation of Solution
In the given molecular model,
Three atoms are bonded to the central atom. The angle around the central atom is around
Therefore,
The geometry of the given molecular model is trigonal planar.
Want to see more full solutions like this?
Chapter 4 Solutions
Study Guide And Full Solutions Manual For Fundamentals Of General, Organic, And Biological Chemistry
- What structures are labeled 1 in the diagrams? What structure is labeled 2 in the diagrams? What is labeled 3 in both diagrams? What is labeled 4 in the diagrams? What is labeled 5 in the diagrams?arrow_forwardWhich of the following structures exhibit geometric isomerism? Draw and name the two in each case.arrow_forwardThe much-abused drug cocaine is an alkaloid. Alkaloids are noted for their bitter taste, an indication of their basic properties. Cocaine, C17H21O4N, is soluble in water to the extent of 0.17g/100mL solution, and a saturated solution has a pH = 10.08. What is the value of Kb for cocaine?arrow_forward
- Convert the following structural formulas into condensed structures.arrow_forwardDHA is a fatty acid derived from fish oil and an abundant fatty acid invertebrate brains. Hydrogenation of DHA forms docosanoic acid[CH3(CH2)20CO2H], and ozonolysis forms CH3CH2CHO, CH2(CHO)2 (fiveequivalents), and HCOCH2CH2CO2H. What is the structure of DHA if all double bonds have the Z configuration?arrow_forwardIdentify all of the chirality centers in the structure. The chirality centers are: A В C D `NH E F OH b H Он I J K L M N P Q Rarrow_forward
- The structure shown is an example of a type of macromolecule (carbohydrate, lipid, protein, or nucleic acid) that is an important biological polymer. Identify the type of macromolecule macromolecule in the cell. H H H NH H H H H H N|H shown, the type of monomer it is made from, how you came to your conclusion and the general types of important biological functions of this type of 2=0 N-H -O-Harrow_forwardHow many peptide bonds were produced by creating the following How many H2O particle were produced by creating the following structure if the starting reagent is glucose? * structure if the starting reagent is glucose? * CH,OH CH,OH CH,OH CH;OH CH;OH CH;OH OH он он OH 250 ÓH OH OH ÓH OH OH 300 А.) 125 B.) 250 А.) 30 c.) 25 B.) 301 D.) 500 C.) 302 E.) O D.) 300 E.) 150 F.) Oarrow_forwardCompound P was discovered by a scientist. Compound P is a dipeptide, optically active and has the molecular formula C„H14N2O3. Compound P is formed when compound Q and compound R joined together by condensation reaction. While, monomers S and T are formed by modifying the compounds Q and R. Polymer U is formed by the condensation reaction of monomers S and T. Draw the possible structural formulae of compounds P, Q, R, S, T and U. Label the peptide bond(s) for compound P. Draw the possible structural formulae for repeating unit of polymer U. Please state the number of functional groups present in compound P.arrow_forward
- A mixture of proteins contains four different polypeptides, all in ~equal concentration, in solution with the following properties: Protein Molecular Mass (kDa) Isoelectric point A 45 4.5 B 77 6.0 C 28 4.1 D 14 10.7 A fraction of the protein solution is applied to a strong cation exchange column using a buffer at pH 8.0 with increasing [NaCl] from 0.05 M – 1.0 M. The chromatogram is shown below: 1. Based on the data presented, which of the following statements is true: Peak #1 is protein D Peak #4 is protein C Peak #3 is protein A Peak #4 is protein D Peak #2 is protein B 2. Since you know that the proteins are all present in approximately equal concentrations, the different relative peak areas tell you that: There are more neutral amino acids in protein #4…arrow_forwardUse Frost Circles to complete the molecular orbital diagram for cyclooctatetrane. Label the bonding, non bonding, and anti bonding MO’s. If the molecule is planar, would it be aromatic, antiaromatic, or nonaromatic? If the molecule is nonplanar, would it be aromatic, antiaromatic, or nonaromatic?arrow_forwardA) Refer to the figure below, Identify and explain the two types of reactions, and describe what are the importance of these reactions to our bodies. Reaction 1 H Monomer Reaction 2 H H OH + H H H₂O H H₂O OH + H H OH OH + H H₂O H₂O OH OH + H OH OH OH OH B) What are the main chemical interactions that determine and maintain the quaternary structure of proteins. Also, what are the conditions that can alter these interactions? [arrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





