
Concept explainers
Fill in the blanks in each line in the following table. The first line is already completed as an example.
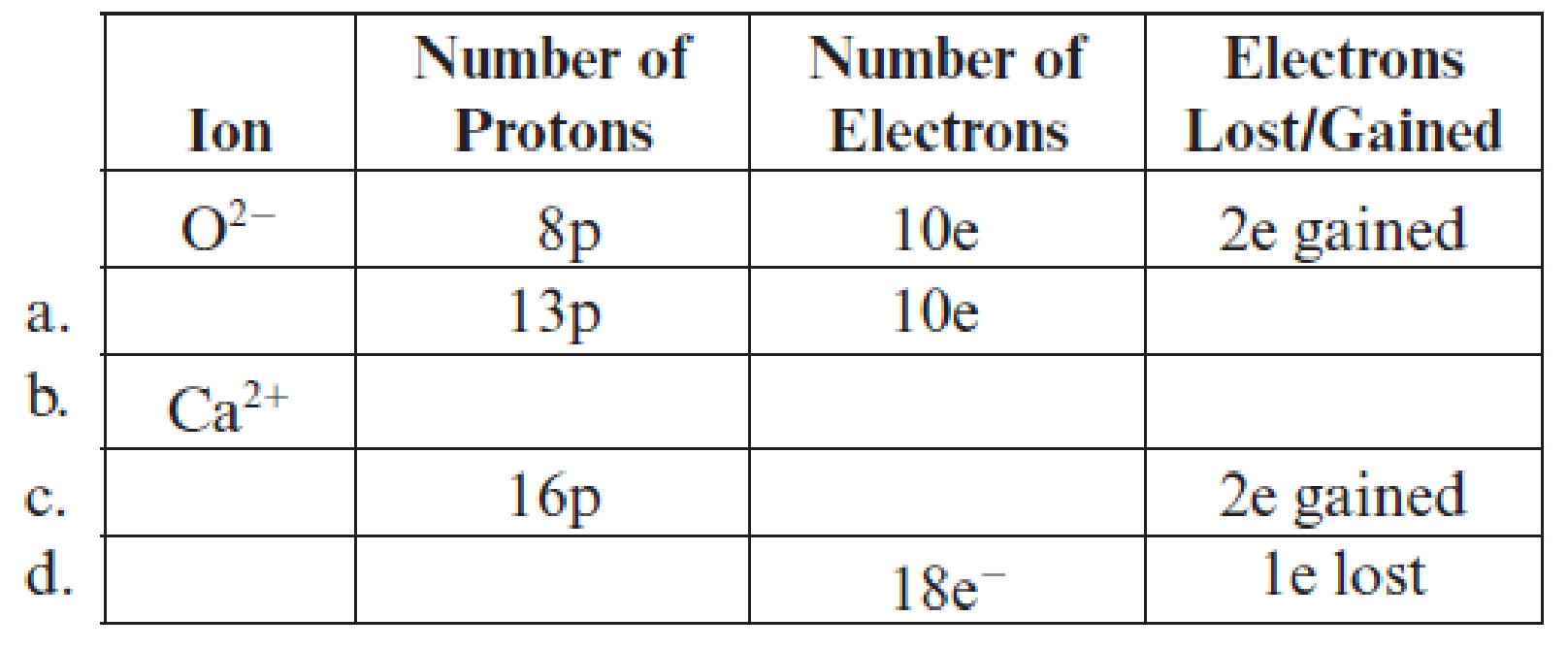
(a)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.28EP
Complete table is shown below:
Explanation of Solution
The charge on an ion and its magnitude is equal to the number of protons minus the number of electrons.
The given ion has
Hence, the symbol of ion is
(b)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.28EP
Complete table is shown below:
Explanation of Solution
The charge on an ion and its magnitude is equal to the number of protons minus the number of electrons.
The atomic number of calcium is
Hence, the number of protons are
(c)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom forms ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.28EP
Complete table is shown below:
Explanation of Solution
The charge on an ion and its magnitude is equal to the number of protons minus the number of electrons.
The ion has
Hence, ion is
(d)
Interpretation:
Filling of each blank in the following table has to be done:
Concept Introduction:
Atoms are composed of three types of particles called subatomic particles. They are as follows:
- Protons: Positively charged particles in an atom.
- Neutrons: Neutral charged particles in an atom.
- Electrons: Negatively charged particles in an atom.
The neutral atom has equal number of protons and electrons. Gaining or loosing of electrons of an atom form ion.
Negative charged ions are formed by gaining one or more electrons and it has more electrons than protons.
Positive charged ions are formed by losing one or more electrons and it has more protons than electrons.
Answer to Problem 4.28EP
Complete table is shown below:
Explanation of Solution
The charge on an ion and its magnitude is equal to the number of protons minus the number of electrons.
The number of electrons in ion is
Potassium has
Hence, ion is
Want to see more full solutions like this?
Chapter 4 Solutions
CHM 104/201 <C>
- Fill in the blanks in each line of the following table that involves the representative elements X and Y. The first line is already completed as an example.arrow_forwardComplete the following table by filling in the blanks in each row. The first row has been completed as an example.arrow_forwardComplete the following table to predict whether the given atom will gain or lose electrons in forming the ion most likely to form when in ionic compounds. Atom Gain (G) or Lose (L) Electrons Ion Formed K Cs Br S Searrow_forward
- What would be the chemical symbol for an ion with each of the following numbers of protons and electrons? a. 20 protons and 18 electrons b. 8 protons and 10 electrons c. 7 protons and 10 electrons d. 4 protons and 2 electronsarrow_forwardWhat are bus? How are ions formed from atoms? Do isolated atoms form ions spontaneously? To what do the termscationandanionrefer? In terms of subatomic particles, how is an ion related to the atom from which it is formed? Does the nucleus of an atom change when the atom is converted into an ion? How can the periodic table be used to predict what ion an element’s atoms will form?arrow_forwardDistinguish between the following terms. a. molecule versus ion b. covalent bonding versus ionic bonding c. molecule versus compound d. anion versus cationarrow_forward
- Distinguish between the following terms. a. molecule versus ion b. covalent bonding versus ionic bonding c. molecule versus compound d. anion versus cationarrow_forwardWrite chemical formulas for the following binary ionic compounds. a. Iron(III) oxide b. Iron(II) oxide c. Nickel(III) sulfide d. Copper(I) bromidearrow_forwardWrite chemical formulas for the following binary ionic compounds. a. Gallium nitride b. Zinc chloride c. Magnesium sulfide d. Aluminum nitridearrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





