
Concept explainers
(a)
Interpretation:
The
Concept introduction:
Answer to Problem 4.47AP
The configuration assigned to the given structure is
Explanation of Solution
The
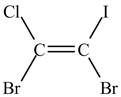
Figure 1
In this case, bromide ion gets priority over chloride ion and iodide ion gets priority over chloride ion. The structure with higher priority order is written as shown below.
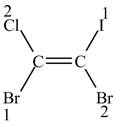
Figure 2
The higher priority groups are present on opposite sides of the double bond. As a result,
The configuration of the given structure is
(b)
Interpretation:
The
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
Answer to Problem 4.47AP
The configuration assigned to the given structure is
Explanation of Solution
The
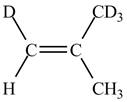
Figure 3
In this case, the
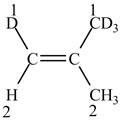
Figure 4
The higher priority atoms are present on the same side of the double bond. As a result,
The configuration of the given structure is
(c)
Interpretation:
The
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
Answer to Problem 4.47AP
The configuration assigned to the given structure is
Explanation of Solution
The
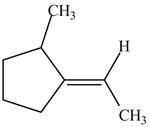
Figure 5
In this case, right side of double bond contains
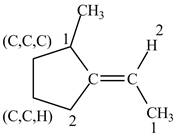
Figure 6
One carbon atom is attached to three carbon atoms and other attached to two carbon atoms. Therefore, the carbon atom which is attached to three carbon atoms gets higher priority. As a result, higher priority atoms are present on opposite sides of the double bond. The structure is assigned
The configuration of the given structure is
(d)
Interpretation:
The
Concept introduction:
Alkenes are the unsaturated class of organic compounds which have a double bond in their structure. The general formula of alkene is written as
Answer to Problem 4.47AP
The configuration assigned to the given structure is
Explanation of Solution
The
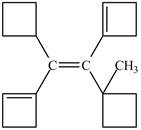
Figure 7
In this case, left and right side of the double bond contains a carbon atoms cyclic ring whose priority order is identified on the basis of higher priority order atoms attached to its carbon atom which is marked as shown below.
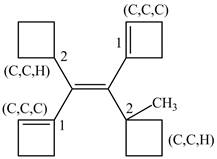
Figure 8
On both sides, One carbon atom is attached to three carbon atoms and other attached to two carbon atoms. Therefore, the carbon atom which is attached to three carbon atoms gets higher priority. Along with double bond get higher priority over the single bond. As a result, higher priority atoms are present on opposite sides of the double bond. The structure is assigned
The configuration of the given structure is
Want to see more full solutions like this?
Chapter 4 Solutions
ORGANIC CHEMISTRY SAPLING ACCESS + ETEX
- A compound C4H11N is known from its reactivity andspectroscopic properties to have no hydrogen atomsattached directly to the nitrogen atom. Write all structuralformulas consistent with this informationarrow_forward(a) Indicate the following alkenes in order of increasing stability. Justify your answers.arrow_forwardWrite chemical structures for compounds A through D in the following sequence of reac- tions. Compounds A and C are alcohols, one of which is cyclicarrow_forward
- Provide a detailed, stepwise mechanism for the following transformation. Use the curved arrow formalism to show the flow of electrons. Show all lone pairs, formal charges, etcarrow_forwardGive the series of reactions below, identify and give the iupac name for the following compounds, in short identify and name A,B,Carrow_forwardFor the molecule given, prioritize each substituent on the stereogenic carbon, and assign absolute stereochemistry.arrow_forward
- Compound A and compound B are in equilibrium. Write a stepwise mechanism from compound Ato compound B showing ALL intermediates. Use curved arrows to symbolize the flow of electrons to show how each of the intermediates and products are formed. Show all lone pairs and formal charges. Lastly, explain which compound (Aor B) will be in higher concentration.arrow_forwardExplain the concept of Hydrogenation Data and Degrees of Unsaturation ?arrow_forwardExplain the Mechanism - Addition of Dichlorocarbene to an Alkene ?arrow_forward
- Give the reagents and conditions necessary for the following conversion. A to B, B to D, B to C, B to E, E to F, E to G, G to H, H to I. Hence deduce the name and structural formula of the compounds C and I. Compare the procedure for converting F and E to G.arrow_forwardGive the structural chemistry of active methylene, classify it asradical/intermediate/stable organic compound, reagent of its production and role in alkylationarrow_forwardCompound F may be synthesised by the method attached: Draw the structural formulas of compounds A, C, D, E and F in the boxes providedarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

