
(a)
Interpretation:
The number of elements that forms a negatively charged ions among the following highlighted elements in the below periodic table has to be identified.
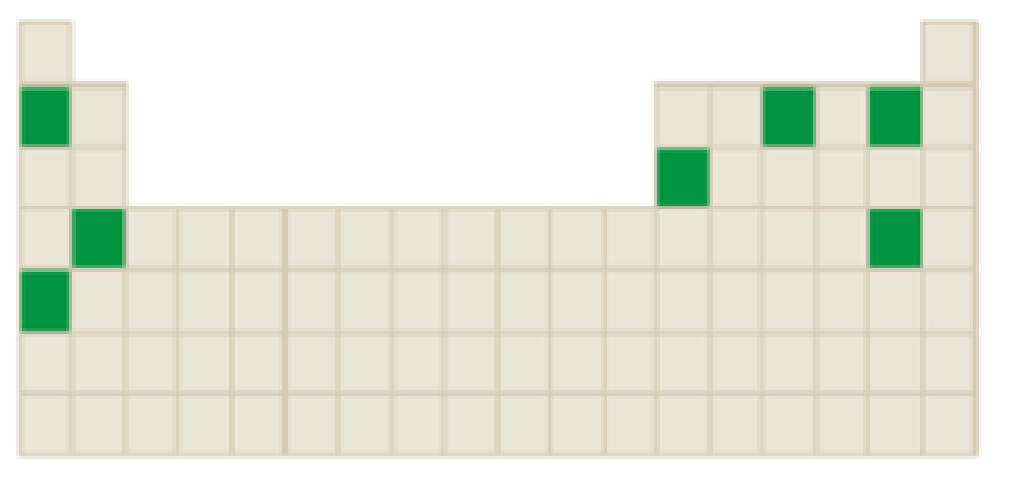
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
(b)
Interpretation:
The elements that forms ions through gain of electrons among the following highlighted elements in the below periodic table has to be identified.
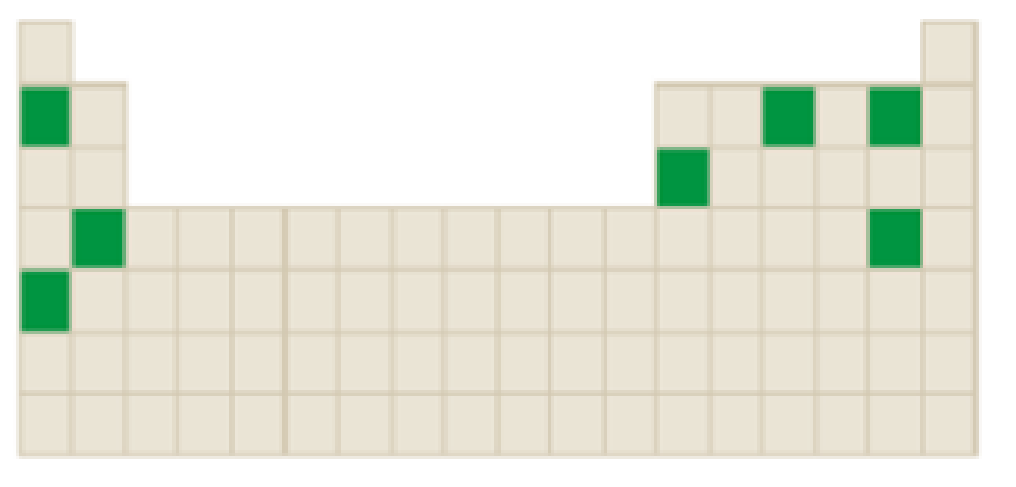
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
(c)
Interpretation:
The elements which forms ions that has a charge magnitude of
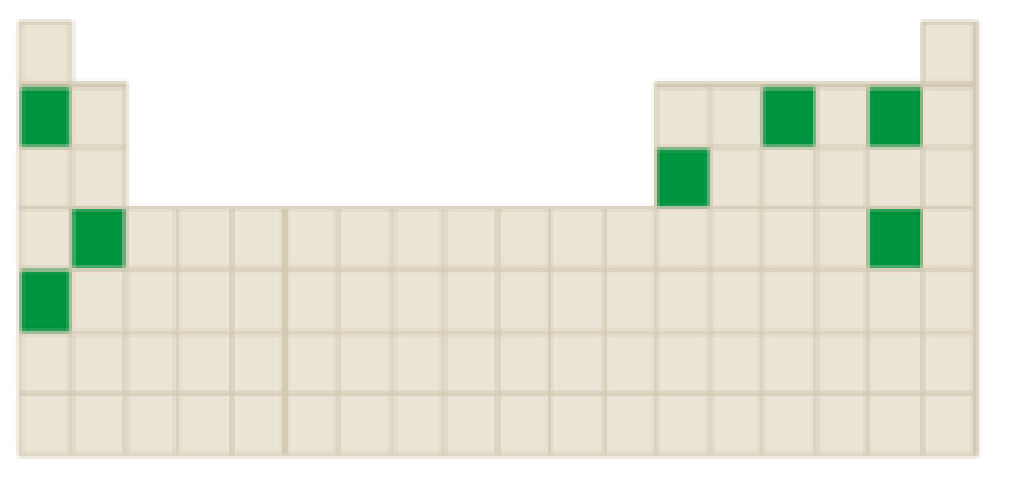
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
(d)
Interpretation:
The elements that forms an ion that involves loss of two or more electrons among the following highlighted elements in the below periodic table has to be identified.
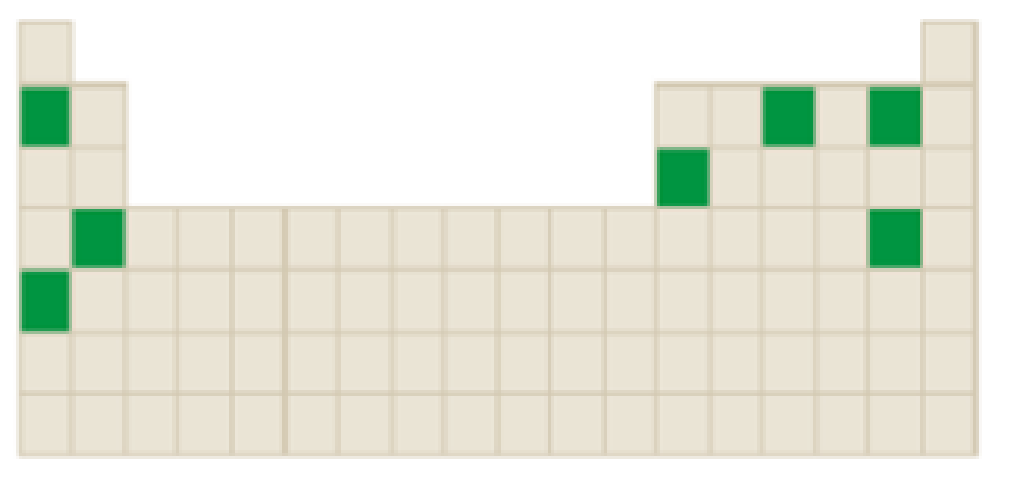
Concept Introduction:
Atoms tend to gain or lose electrons until they obtain an electron configuration that is the same as that of a noble gas.
The neutral atom has equal number of protons and electrons. Gaining or loosing of a electrons of an atoms form ions.
Atoms form their ions to attain noble gas configuration.
- Group IA, IIA and IIIA are metal atoms containing one, two or three valence electrons. These metal atoms lose their valence electrons to get noble gas configuration.
- Group VA, VIA VIIA are non-metal atoms containing five, six or seven valence electrons. These non-metal atoms acquire electrons to get noble gas configuration.
- Group IVA group elements have four valence electrons. These elements either gain or lose their electrons to get noble gas configuration.
Trending nowThis is a popular solution!

Chapter 4 Solutions
Bundle: General, Organic, and Biological Chemistry, 7th + OWLv2 Quick Prep for General Chemistry, 4 terms (24 months) Printed Access Card
- What are bus? How are ions formed from atoms? Do isolated atoms form ions spontaneously? To what do the termscationandanionrefer? In terms of subatomic particles, how is an ion related to the atom from which it is formed? Does the nucleus of an atom change when the atom is converted into an ion? How can the periodic table be used to predict what ion an element’s atoms will form?arrow_forwardWrite a symbol for each of the following ions: a.A bromine atom that has gained one electron b.A sodium atom that has lost one electron c.A sulfur atom that has gained two electronsarrow_forwardWhat is the difference between sulfuric acid and hydrosulfuric acid?arrow_forward
- Fill in the blanks with the smallest integers possible. When gallium (Z=31) reacts with sulfur to form an ionic compound, each metal atom loses ______ electrons and each nonmetal gains_____electronss. There must be _____ gallium atoms for every _____sulfur atoms in the reaction.arrow_forwardEach of the following Lewis symbols represents a Period 2 element. Determine each elements identity.arrow_forwardDoes a cation gain protons to form a positive charge or does it lose electrons?arrow_forward
- What is apolyatomicion? Give examples of five common polyatomic ions.arrow_forwardComplete the following table to predict whether the given atom will gain or lose electrons in forming the ion most likely to form when in ionic compounds. Atom Gain (G) or Lose (L) Electrons lon Formed K Cs Br S Searrow_forwardThis subatomic element has a charge of -1 and no mass The unequal sharing of electrons in covalent bonds form _____ molecules, such as water During _____ bonding electrons are shared between atoms. The ability of water molecules to form _____ bonds accounts for the unique properties of water. An atom that has lost or gained an electron is called?arrow_forward
- 15. What is the basic form for the names of ionic compounds containing a metal that forms more than one type of ion?arrow_forward1.How are the atoms in ionic compounds held together? a) The ions in ionic compounds form crystal lattices. Explain what this means. b) How do ionic compounds appear to our eye? Why do ionic compounds have high melting and boiling points? Explain why a salt crystal will shatter if you hit it with a hammer. Explain why ionic compounds will conduct electricity when they dissolve in water.arrow_forwardIN IN IN Polyvalent metal ions. Determine the charge of the transition metal, post transition metal, lanthanide metal, or actinide metal the ionic compound. The chemical symbol for the metal is bolded in red for clarity. Ionic compounds must be electrically neutral. Type the charges of the metal ions into the boxes next to their chemical formulae. 1+ 1. Fe₂O3 FeO ScBr3 GeS₂ MoO; 6. CEF4 7. 8. 2. 3. 5. 2+ 3+ 4+ 5+ 6+ 9. CHEMISTRY Polyvalent Metal Ions PdO PdO₂ NIS 10. Agl 11. SnCl₂F₂ 12. Nb₂0s 13. CoCl3 14. NiBr₂ 15. EuBr₂Cl WO3 PtF4 AuCl AuCl; V₂Ss 16. 17. 18. 19. 20.arrow_forward
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





