
Concept explainers
Rank the following ions in order of increasing number of unpaired electrons: Tr3+, Fe2+, V3+, Cu+, Mn4+.
Interpretation: It should be ranked the given set of ions in increasing order of number of unpaired electrons.
Concept Introduction:
- Aufbau Principle tells that the orbital with the lower energy is filled with electrons first and then the filling of higher energy orbital follows. By using this, valence orbital diagram can be draw for any atoms or ions.
- Electronic configuration is the arrangement of the electrons of atoms in the orbital. The electronic configurations are writing for atoms and ions, in accordance with Pauli Exclusion Principle and Hund’s rule.
- According to Pauli Exclusion Principle, no two electrons having the same spin can occupy the same orbital.
- According to Hund’s rule, the orbital in the subshell is filled singly by one electron before the same orbital is doubly filled. When the orbital is singly filled, all the electrons have same spin. In a doubly filled orbital, there are two electrons with opposite spin.
To determine the increasing order of unpaired electrons present in the given set of ions.
Answer to Problem 4.79QP
Ions in increasing order of unpaired electrons,
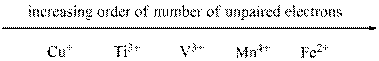
Explanation of Solution
Electronic configuration of
The valence orbital diagram of
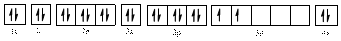
The electronic configuration of
Electronic configuration of
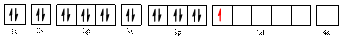
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there is one unpaired electron and which is highlighted in red colour.
Electronic configuration of
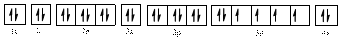
The electronic configuration of
Electronic configuration of
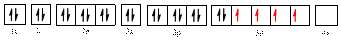
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are four unpaired electrons that are highlighted in red colour.
Electronic configuration of
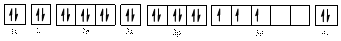
The electronic configuration of
Electronic configuration of
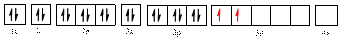
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are two unpaired electrons, that are highlighted in red colour.
Electronic configuration of
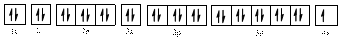
The electronic configuration of
Electronic configuration of
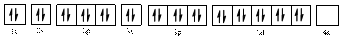
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this we can find that there are no unpaired electrons present.
Electronic configuration of
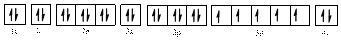
The electronic configuration of
Electronic configuration of
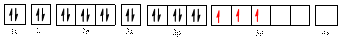
The electronic configuration of
The valence orbital diagram is drawn as shown above. From this, we can find that there are three unpaired electrons present and the same is highlighted in red colour.
Given ions were ranked in increasing order of unpaired electron as follows,
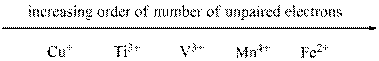
From the number of unpaired electrons for each of the given ions found out in the previous steps we can arrange them in increasing order of number of unpaired electrons as shown above.
The given sets of ions were ranked in increasing order of number of unpaired electrons.
Want to see more full solutions like this?
Chapter 4 Solutions
CHEMISTRY: ATOMS FIRST VOL 1 W/CONNECT
- Write the ground state electron configuration for (a) Mg, Mg2+(b) N, N3- (c) Ti, Ti4+ (d) Sn2+ Sn4+arrow_forwardHow many unpaired electrons are there 111 the following ions? (a) V3+(b) Sn4+(c) I-(d) W4+arrow_forwardWrite the electron configurations far each of the following elements: (a) Sc. (b) Ti. (c) Cr. (d) Fe. (e) Ruarrow_forward
- Which of the following has two unpaired electrons? (a) Mg (b) Si (c)S (d) Both Mg and S (e) Both Si and S.arrow_forwardWrite electron configurations for the following elements. a. The Group III A element in the same period as 4Be b. The Period 3 element in the same group as 5B c. The lowest-atomic-numbered metal in Group IIA d. The two Period 3 elements that have no unpaired electronsarrow_forwardUse partial atomic orbital box diagrams to explain the number of unpaired electrons shown in Figure 20.2 for (a) Mn2+ and (b) Cr3+.arrow_forward
- The electron affinity of the lutetium atom (element 71) was measured using the technique of photoelectron spectroscopy with an infrared laser (the essay on p. 310 describes this instrumental method, using X rays). In this experiment, a beam of lutetium negative ions, Lu, was prepared and irradiated with a laser beam having a wavelength at 1064 nm. The energy supplied by a photon in this laser beam removes an electron from a negative ion, leaving the neutral atom. The energy needed to remove the electron from the negative ion to give the neutral atom (both in their ground states) is the electron affinity of lutetium. Any excess energy of the photon shows up as kinetic energy of the emitted electron. If the emitted electron in this experiment has a kinetic energy of 0.825 eV, what is the electron affinity of lutetium?arrow_forwardGive the symbol of the main-group metals in period 4 with the following number of unpaired electrons per atom. (Transition metals are not included.) (a) 0(b) 1(c) 2(d) 3arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





