
Consider two chemical changes: one occurring at a tetrahedral
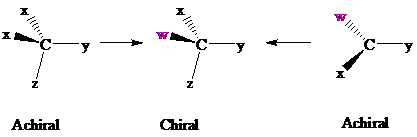
Both transformations convert C in each achiral reactant to a chirality center in the product. The two achiral reactants are classified as prochiral. C is a prochiralitycenter in
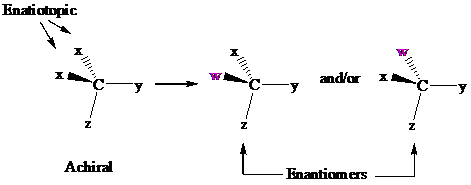
In achiral molecules with tetrahedral prochiralitycenters, substitution of one of the two x groups by w gives the enantiomer of the product that results from substitution of the other. The two x groups occupy mirror-image sites and are enantiotopic.
Enantiotopic groups are designated as pro-R or pro-S by a modification of Cahn–Ingold– Prelog notation. One is assigned a higher priority than the other without disturbing the priorities of the remaining groups, and the R,S configuration of the resulting chirality center is determined in the usual way. If it is R, the group assigned the higher rank is pro-R. If S, this group is pro-S. Ethanol and citric acid illustrate the application of this notation to two prochiral molecules.
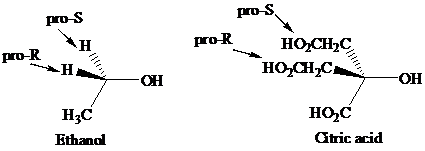
Citric acid played a major role in the development of the concept of prochirality. Its two
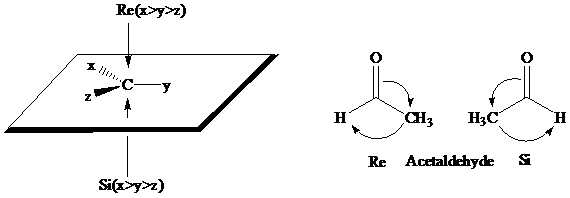
The stereochemical aspects of many enzyme-catalyzed reactions have been determined. Then enzyme alcohol dehydrogenase catalyzes the oxidation of ethanol to acetaldehyde by removing the pro-R hydrogen (abbreviated as HR). When the same enzyme catalyzes the reduction of acetaldehyde to ethanol, hydrogen is transferred to the Re face.
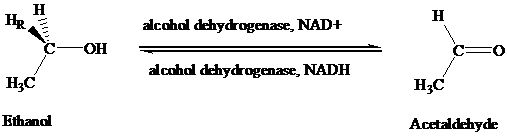
The enzyme fumarasecatalyzes the addition of water to the double bond of fumaric acid.
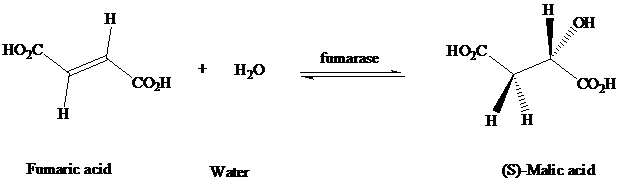
The
A. syn Addition
B. anti Addition
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Organic Chemistry - Standalone book
- Write the name of the following compounds. Which of them are stereoisomers? Which of them are optically active? Use cis-trans, (E)(Z) nomenclature and assign the absolute configuration (R),(S), if any to each chiral center.arrow_forward(a) Draw the nine isomers having the formula C7H16 . (Hint: There is one structure with a seven-carbon chain, two structures with six-carbon chains [one is illustrated in the margin above], five structures with a five-carbon chain, and one structure with a fourcarbon chain.)(b) Identify the isomers of C7H16 that are chiral.arrow_forwardOrganic Chemistry HW: 2,6-dimethyloct-2-ene cannot be handwritten, please type or use a program to draw Chiral Carbons show the expanded structure of this molecule. Determine if this molecule contains any chiral carbons. If there are chiral carbons in the molecule, circle or highlight all of them. If your molecule does not contain any chiral carbons explain why none of the carbons are chiralarrow_forward
- A well known non steroidal anti inflammation drug NSAID exists in two sterochemical forms. Only one is biologically active. Below is the structure of the biological active form. Identify the chiral center and determine if it is the R- or S- stereoisomer.arrow_forwardCholesterol is one of the important components of the animal cell membrane. Label the circled chiral centers as R/S, and circle the rest of the chiral centers in this compound.arrow_forwardDesignate the chiral centers of the following molecules and show which one of them is achiral?arrow_forward
- Consider molecules with more than one chiral center. Consider 2,3-dyhdroxybutanoic acid. a. How many chiral centers does it have? b. Which ones? c. Reproduce the structure and encircle the four groups, which are linked to each chiral carbon.arrow_forwardThe quantitative differences in biological activity between the two enantiomers of a compound are sometimes quite large. For example, the D isomer of the drug isoproterenol, used to treat mild asthma, is 50 to 80times more effective as a bronchodilator than the L isomer. Identify the chiral center in isoproterenol. Why do the two enantiomers have such radically different bioactivity?arrow_forwardHow many chiral centers are in each of the molecules and how to find them?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning

