
Each of the following ground-state electron configurations represents one or more of the transition metal ions in Figure 4.12. Identify the ion or ions represented by each.
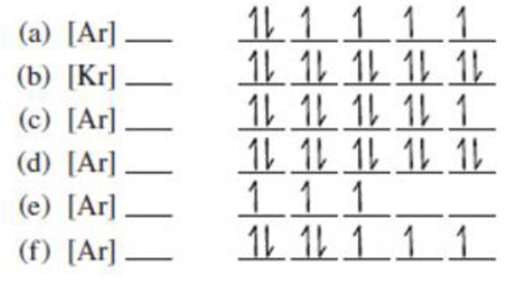
(a)
Interpretation: The ion or ions of transition metals that is represented by the given ground state electronic configuration has to be identified.
Concept Introduction:
- The d-block elements in the periodic table are known as transition metals. When looking at the first series of d-block elements the 4s orbital is filled first before the filling of 3d orbital happens. Therefore, when electron is removed from d-block elements, the two electrons from the “s” orbital is removed first followed by the “d” orbital. This is the reason why many of the transition metals form ions in “+2” state. Two possible oxidation states are there for transition metals namely “+2” and “+3”.
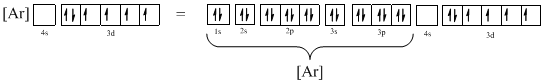
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The transition metal ion or ions that represent the given ground-state electronic configuration.
Answer to Problem 4.85QP
Answer
The transition metal ion found for (a) is
Explanation of Solution
Ground-state electronic configuration of the given ion (a) is,
The atomic ground-state configuration is given in the problem statement. From this we can find that the given ion has an empty 4s orbital and 3d sub-shell that has six electrons.
Identifying the ions as follows,
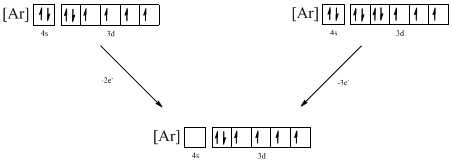
From the configuration given in the problem statement, we can conclude that the ion is formed by removing two electrons from 4s orbital. Another ion can be formed by removing electron from 4s orbital and 3d orbital. Therefore there are two possibility for the ions to have the given electron configuration. It is found to be
(b)
Interpretation: The ion or ions of transition metals that is represented by the given ground state electronic configuration has to be identified.
Concept Introduction:
- The d-block elements in the periodic table are known as transition metals. When looking at the first series of d-block elements the 4s orbital is filled first before the filling of 3d orbital happens. Therefore, when electron is removed from d-block elements, the two electrons from the “s” orbital is removed first followed by the “d” orbital. This is the reason why many of the transition metals form ions in “+2” state. Two possible oxidation states are there for transition metals namely “+2” and “+3”.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The transition metal ion or ions that represent the given ground-state electronic configuration.
Answer to Problem 4.85QP
Answer
The transition metal ion found for (b) is
Explanation of Solution
Ground-state electronic configuration of the given ion (b) is,

The atomic ground-state configuration is given in the problem statement. From this we can find that the given ion has an empty 5s orbital and 4d sub-shell that has ten electrons.
Identifying the ion as follows,
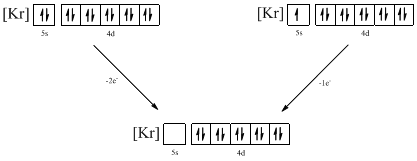
From the configuration given in the problem statement, we can conclude that the ion is formed by removing two electrons from 5s orbital. Another possibility is that the 5s orbital may be singly filled. Therefore, there are two possibility of ions to have the given ground-state electronic configuration. It is found to be
(c)
Interpretation: The ion or ions of transition metals that is represented by the given ground state electronic configuration has to be identified.
Concept Introduction:
- The d-block elements in the periodic table are known as transition metals. When looking at the first series of d-block elements the 4s orbital is filled first before the filling of 3d orbital happens. Therefore, when electron is removed from d-block elements, the two electrons from the “s” orbital is removed first followed by the “d” orbital. This is the reason why many of the transition metals form ions in “+2” state. Two possible oxidation states are there for transition metals namely “+2” and “+3”.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The transition metal ion or ions that represent the given ground-state electronic configuration.
Answer to Problem 4.85QP
Answer
The transition metal ion found for (c) is
Explanation of Solution
Ground-state electronic configuration of the given ion (c) is,
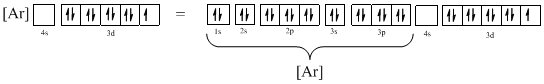
The atomic ground-state configuration is given in the problem statement. From this we can find that the given ion has an empty 4s orbital and 3d sub-shell that has nine electrons.
Identifying the ion as follows,
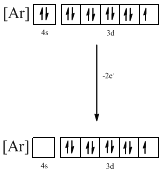
From the configuration given in the problem statement, we can conclude that the ion is formed by removing one electron from 4s orbital and one electron form 3d orbital. The ion that has the given ground-state electronic configuration is found to be
(d)
Interpretation: The ion or ions of transition metals that is represented by the given ground state electronic configuration has to be identified.
Concept Introduction:
- The d-block elements in the periodic table are known as transition metals. When looking at the first series of d-block elements the 4s orbital is filled first before the filling of 3d orbital happens. Therefore, when electron is removed from d-block elements, the two electrons from the “s” orbital is removed first followed by the “d” orbital. This is the reason why many of the transition metals form ions in “+2” state. Two possible oxidation states are there for transition metals namely “+2” and “+3”.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The transition metal ion or ions that represent the given ground-state electronic configuration.
Answer to Problem 4.85QP
Answer
The transition metal ion found for (d) is
Explanation of Solution
Ground-state electronic configuration of the given ion (d) is,
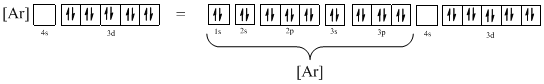
The atomic ground-state configuration is given in the problem statement. From this we can find that the given ion has an empty 4s orbital and 3d sub-shell that has ten electrons.
Identifying the ion as follows,
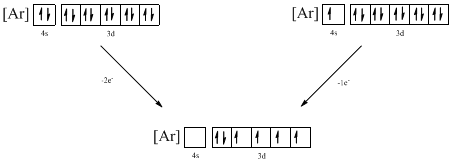
From the configuration given in the problem statement, we can conclude that the ion is formed by removing one electron from 4s orbital. Another possibility is that two electrons can be removed from the 4s orbital. Therefore, there are two possibility of ions to have the given ground-state electronic configuration. It is found to be
(e)
Interpretation: The ion or ions of transition metals that is represented by the given ground state electronic configuration has to be identified.
Concept Introduction:
- The d-block elements in the periodic table are known as transition metals. When looking at the first series of d-block elements the 4s orbital is filled first before the filling of 3d orbital happens. Therefore, when electron is removed from d-block elements, the two electrons from the “s” orbital is removed first followed by the “d” orbital. This is the reason why many of the transition metals form ions in “+2” state. Two possible oxidation states are there for transition metals namely “+2” and “+3”.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The transition metal ion or ions that represent the given ground-state electronic configuration.
Answer to Problem 4.85QP
Answer
The transition metal ion found for (e) is
Explanation of Solution
Ground-state electronic configuration of the given ion (e) is,
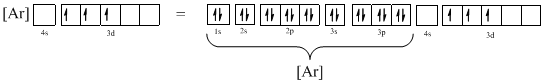
The atomic ground-state configuration is given in the problem statement. From this we can find that the given ion has an empty 4s orbital and 3d sub-shell that has three electrons.
Identifying the ion as follows,
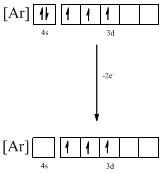
From the configuration given in the problem statement, we can conclude that the ion is formed by removing two electrons from 4s orbital. Therefore, there is only one possibility for the given ground-state electronic configuration. The ion is found to be
(f)
Interpretation: The ion or ions of transition metals that is represented by the given ground state electronic configuration has to be identified.
Concept Introduction:
- The d-block elements in the periodic table are known as transition metals. When looking at the first series of d-block elements the 4s orbital is filled first before the filling of 3d orbital happens. Therefore, when electron is removed from d-block elements, the two electrons from the “s” orbital is removed first followed by the “d” orbital. This is the reason why many of the transition metals form ions in “+2” state. Two possible oxidation states are there for transition metals namely “+2” and “+3”.
- For simpler representation of ions or atoms, the electronic configuration of the completed octet noble gas configuration is considered and the remaining orbital alone is shown explicitly. The ground-state configuration of the noble gases are given below,
To identify: The transition metal ion or ions that represent the given ground-state electronic configuration.
Answer to Problem 4.85QP
Answer
The transition metal ion found for (f) is
Explanation of Solution
Ground-state electronic configuration of the given ion (f) is,
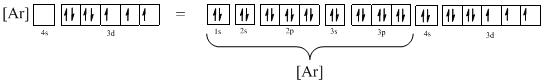
The atomic ground-state configuration is given in the problem statement. From this we can find that the given ion has an empty 4s orbital and 3d sub-shell that has seven electrons.
Identifying the ion as follows,
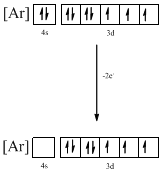
From the configuration given in the problem statement, we can conclude that the ion is formed by removing two electrons from 4s orbital. Therefore, there is only one possibility for the given ground-state electronic configuration. The ion is found to be
Want to see more full solutions like this?
Chapter 4 Solutions
CHEMISTRY: ATOMS FIRST ALEKS CODE
- Use atomic orbital box diagrams to determine which chromium ground-state configuration has the greater number of unpaired electrons: [Ar] 3d44s2 or [Ar] 3d54s1.arrow_forwardWrite electron configurations for the following elements. a. The Group III A element in the same period as 4Be b. The Period 3 element in the same group as 5B c. The lowest-atomic-numbered metal in Group IIA d. The two Period 3 elements that have no unpaired electronsarrow_forward6.84 Which graph correctly depicts the first ionization energy of three elements in groups 14 (dashed line) and 17 (solid line)? Explain the reasoning you used to make your choice.arrow_forward
- Which main group atom would be expected to have the lowest second ionization energy?arrow_forwardRead the labels of several commercial products and identify monatomic ions of at least four transition elements contained in the products. Write the complete electron configurations of these cations.arrow_forwardThe magnet in the following photo is made from neodymium, iron, and boron. A magnet mode of on alloy containing the elements Nd, Fe, and B. (a) Write the electron configuration of each of these elements using an orbital box diagram and noble gas notation. (b) Are these elements paramagnetic or diamagnetic? (c) Write the electron configurations of Nd3+ and Fe3+ using orbital box diagrams and noble gas notation. Are these ions paramagnetic or diamagnetic?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





