
Concept explainers
a.
Interpretation:
The partial charge by symbol 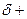 and
and 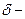 should be indicated on each atom if the
should be indicated on each atom if the 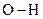 bond is polar covalent.
bond is polar covalent.
Concept introduction: The covalent bond is a type of bond which formed by sharing of electrons between atoms. It forms between two non-metals.
A polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared unequally.
A non-polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared equally.
The relation between elements electronegativity and bonding type are as follows:
| Electro-negativity difference | Bonding type |
| < 0.5 | Non-polar covalent |
| 0.5-2.0 | Polar covalent |
| > 2.0 | Ionic |
The direction of dipole is always from less electronegative atom to more electronegative atom, which indicates the shifting of bonding electrons.
If in 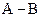 bond, A is more electronegative that B then partial charge will be indicated as follows:
bond, A is more electronegative that B then partial charge will be indicated as follows:
`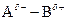
b.
Interpretation:
The partial charge by symbol 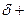 and
and 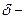 should be indicated on each atom if the
should be indicated on each atom if the 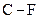 bond is polar covalent.
bond is polar covalent.
Concept introduction: The covalent bond is a type of bond which formed by sharing of electrons between atoms. It forms between two non-metals.
A polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared unequally.
A non-polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared equally.
The relation between elements electronegativity and bonding type are as follows:
| Electro-negativity difference | Bonding type |
| < 0.5 | Non-polar covalent |
| 0.5-2.0 | Polar covalent |
| > 2.0 | Ionic |
The direction of dipole is always from less electronegative atom to more electronegative atom, which indicates the shifting of bonding electrons.
If in 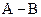 bond, A is more electronegative that B then partial charge will be indicated as follows:
bond, A is more electronegative that B then partial charge will be indicated as follows:
`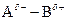
c.
Interpretation:
The partial charge by symbol 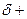 and
and 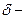 should be indicated on each atom if the
should be indicated on each atom if the 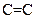 bond is polar covalent.
bond is polar covalent.
Concept introduction: The covalent bond is a type of bond which formed by sharing of electrons between atoms. It forms between two non-metals.
A polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared unequally.
A non-polar covalent bond is a type of bond where the bonded electrons of covalent bond are shared equally.
The relation between elements electronegativity and bonding type are as follows:
| Electro-negativity difference | Bonding type |
| < 0.5 | Non-polar covalent |
| 0.5-2.0 | Polar covalent |
| > 2.0 | Ionic |
The direction of dipole is always from less electronegative atom to more electronegative atom, which indicates the shifting of bonding electrons.
If in 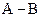 bond, A is more electronegative that B then partial charge will be indicated as follows:
bond, A is more electronegative that B then partial charge will be indicated as follows:
`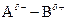
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





