
Chemistry For Changing Times (14th Edition)
14th Edition
ISBN: 9780321972026
Author: John W. Hill, Terry W. McCreary
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 4, Problem 71P
(a)
Interpretation Introduction
Interpretation:
Lewis structure for 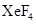 should be drawn.
should be drawn.
Concept introduction:
In order to write Lewis structure of any molecule or ion we follow the simple steps:
- Identify the central atom
- Total sum of the number of valence electrons on each atom in the molecule or ion.
- Arrange a pair of electrons in between two atoms to show a single bond between them.
- Give rest electrons to the atoms to fulfill its octet (in case of hydrogen we go for two electrons only)
- If still electrons are left then place them on a central atom (this is the expansion of valence electron shell)
- If the octet of central atom is not complete, form multiple bonds between the atoms.
(b)
Interpretation Introduction
Interpretation:
Lewis structure for 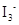 should be drawn.
should be drawn.
Concept introduction:
In order to write Lewis structure of any molecule or ion we follow the simple steps:
- Identify the central atom
- Total sum of the number of valence electrons on each atom in the molecule or ion.
- Arrange a pair of electrons in between two atoms to show a single bond between them.
- Give rest electrons to the atoms to fulfill its octet (in case of hydrogen we go for two electrons only)
- If still electrons are left then place them on a central atom (this is the expansion of valence electron shell)
- If the octet of central atom is not complete, form multiple bonds between the atoms.
(c)
Interpretation Introduction
Interpretation:
Lewis structure for 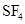 should be drawn.
should be drawn.
Concept introduction:
In order to write Lewis structure of any molecule or ion we follow the simple steps:
- Identify the central atom
- Total sum of the number of valence electrons on each atom in the molecule or ion.
- Arrange a pair of electrons in between two atoms to show a single bond between them.
- Give rest electrons to the atoms to fulfill its octet (in case of hydrogen we go for two electrons only)
- If still electrons are left then place them on a central atom (this is the expansion of valence electron shell)
- If the octet of central atom is not complete, form multiple bonds between the atoms.
(d)
Interpretation Introduction
Interpretation:
Lewis structure for 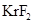 should be drawn.
should be drawn.
Concept introduction:
In order to write Lewis structure of any molecule or ion we follow the simple steps:
- Identify the central atom
- Total sum of the number of valence electrons on each atom in the molecule or ion.
- Arrange a pair of electrons in between two atoms to show a single bond between them.
- Give rest electrons to the atoms to fulfill its octet (in case of hydrogen we go for two electrons only)
- If still electrons are left then place them on a central atom (this is the expansion of valence electron shell)
- If the octet of central atom is not complete, form multiple bonds between the atoms.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry For Changing Times (14th Edition)
Ch. 4 - Prob. 1RQCh. 4 - Prob. 2RQCh. 4 - What are the structural differences among chlorine...Ch. 4 - Prob. 4RQCh. 4 - What are the charges on simple ions formed from...Ch. 4 - Prob. 6RQCh. 4 - In what group of the periodic table would elements...Ch. 4 - Prob. 8RQCh. 4 - Prob. 9RQCh. 4 - Prob. 10P
Ch. 4 - 11. Write Lewis symbols for each of the following...Ch. 4 - Write the Lewis symbol for each species in the...Ch. 4 - Prob. 13PCh. 4 - Prob. 14PCh. 4 - Prob. 15PCh. 4 - Prob. 16PCh. 4 - Prob. 17PCh. 4 - Prob. 18PCh. 4 - Prob. 19PCh. 4 - Prob. 20PCh. 4 - Prob. 21PCh. 4 - Prob. 22PCh. 4 - Prob. 23PCh. 4 - Prob. 24PCh. 4 - There are two common binary ionic compounds formed...Ch. 4 - Prob. 26PCh. 4 - Prob. 27PCh. 4 - Prob. 28PCh. 4 - Prob. 29PCh. 4 - Prob. 30PCh. 4 - Prob. 31PCh. 4 - Prob. 32PCh. 4 - Use Lewis dot symbols to show the sharing of...Ch. 4 - Prob. 34PCh. 4 - Prob. 35PCh. 4 - Prob. 36PCh. 4 - Prob. 37PCh. 4 - Prob. 38PCh. 4 - 39. Supply a formula for the name or a name for...Ch. 4 - Prob. 40PCh. 4 - Prob. 41PCh. 4 - Prob. 42PCh. 4 - Prob. 43PCh. 4 - Prob. 44PCh. 4 - Prob. 45PCh. 4 - Prob. 46PCh. 4 - Prob. 47PCh. 4 - Prob. 48PCh. 4 - Prob. 49PCh. 4 - Prob. 50PCh. 4 - Prob. 51PCh. 4 - Prob. 52PCh. 4 - Classify the bonds in the following as ionic or...Ch. 4 - Prob. 54PCh. 4 - Prob. 55PCh. 4 - Prob. 56PCh. 4 - Prob. 57PCh. 4 - Prob. 58PCh. 4 - Prob. 59PCh. 4 - Prob. 60PCh. 4 - Prob. 61PCh. 4 - Prob. 62PCh. 4 - Prob. 63PCh. 4 - Prob. 64PCh. 4 - Prob. 65PCh. 4 - Prob. 66PCh. 4 - Prob. 67PCh. 4 - Prob. 68PCh. 4 - Prob. 69PCh. 4 - Prob. 70PCh. 4 - Prob. 71PCh. 4 - Prob. 72APCh. 4 - Prob. 73APCh. 4 - Prob. 74APCh. 4 - Prob. 75APCh. 4 - Prob. 76APCh. 4 - Prob. 77APCh. 4 - Prob. 78APCh. 4 - Prob. 79APCh. 4 - Prob. 80APCh. 4 - Prob. 81APCh. 4 - Prob. 82APCh. 4 - Prob. 83APCh. 4 - Prob. 84APCh. 4 - Prob. 85APCh. 4 - Prob. 86APCh. 4 - Prob. 87APCh. 4 - Prob. 88APCh. 4 - Prob. 89APCh. 4 - Prob. 90APCh. 4 - Prob. 91APCh. 4 - Prob. 92APCh. 4 - Prob. 93APCh. 4 - Prob. 4.1CTECh. 4 - Prob. 4.2CTECh. 4 - 4.3 Sodium chloride (NaCI) is a metal-nonmetal...Ch. 4 - Prob. 4.4CTECh. 4 - Prob. 4.5CTECh. 4 - Prob. 4.6CTECh. 4 - Prob. 4.7CTECh. 4 - Prob. 4.8CTECh. 4 - Prob. 4.9CTECh. 4 - Prob. 4.10CTECh. 4 - Prob. 1CGPCh. 4 - Prob. 2CGPCh. 4 - Prepare a PowerPoint, poster, or other...Ch. 4 - Prob. 4CGPCh. 4 - Prob. 5CGPCh. 4 - Prob. 1CHQCh. 4 - Prob. 2CHQCh. 4 - Prob. 3CHQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Calorimetry Concept, Examples and Thermochemistry | How to Pass Chemistry; Author: Melissa Maribel;https://www.youtube.com/watch?v=nSh29lUGj00;License: Standard YouTube License, CC-BY