
(a)
Interpretation:
The systematic name for the following compound should be determined.
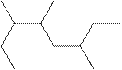
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(b)
Interpretation:
The systematic name for the following compound should be determined.
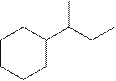
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and whose atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
(c)
Interpretation:
The systematic name for the following compound should be determined.

Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(d)
Interpretation:
The systematic name for the following compound should be determined.
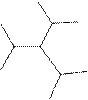
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(e)
Interpretation:
The systematic name for the following compound should be determined.
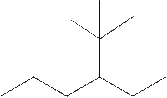
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(f)
Interpretation:
The systematic name for the following compound should be determined.
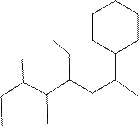
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(g)
Interpretation:
The systematic name for the following compound should be determined.
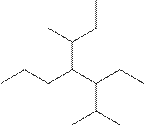
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(h)
Interpretation:
The systematic name for the following compound should be determined.
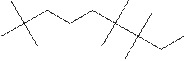
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(i)
Interpretation:
The systematic name for the following compound should be determined.

Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
(j)
Interpretation:
The systematic name for the following compound should be determined.
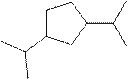
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
The compounds in which a series of atoms are connected to form a ring is known as cyclic compound whereas the compounds which are open chain compounds and whose atoms don't form a ring is known as acyclic compounds. The general molecular formula of a cyclic alkane is
Rules of naming cycloalkanes are:
- First, determine the cycloalkane present in the structure which is considered as a parent chain (maximum number of carbon atoms). If the acyclic alkane chain has more carbon atoms, then the alkyl chain is considered a parent chain.
- For a cyclic system, the number of carbon atoms must be identified as present in different paths connected with two bridgeheads.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group or cycloalkyl group and denote its position on the parent chain with the number.
- The alkyl groups or cycloalkyl groups are written in alphabetical order.
(k)
Interpretation:
The systematic name for the following compound should be determined.
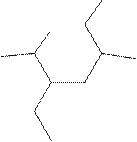
Concept Introduction:
Compounds consisting of carbon and hydrogen are known as hydrocarbons. Saturated hydrocarbon is known as alkane having general molecular formula
Rules of naming alkanes are:
- First, choose the longest continuous chain of carbon atoms known as the parent chain and determines the base name of the alkane.
- The numbering of the parent chain should be done in a way that the substituents get the lowest number.
- The appropriate name should be given to every alkyl group and denote its position on the parent chain with the number.
- The alkyl groups are written in alphabetical order.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
ORGANIC CHEMISTRY 2-SEMESTER-ACCESS
- Give IUPAC names for the following substances:arrow_forwardprovide the correct systemic name for the compound shown herearrow_forward2-chloro-1-phenylpropane is reacted with ammonia, producing amphetamine, a widely misused stimulant. Write the chemical equation for the synthesis of amphetamine as described showing the structure of the compounds.arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning




