
The average temperature of air in 45 min.
Explanation of Solution
Given:
The size of the room
The air pressure in the room remains constant
The initial temperature of the room
The volume of the radiator
The pressure of superheated vapor in radiator
The temperature of superheated vapor
The work input for the fan
The final pressure of the steam
The change in time
Calculation:
Draw the free body diagram of the well-insulated room as shown in figure (1).
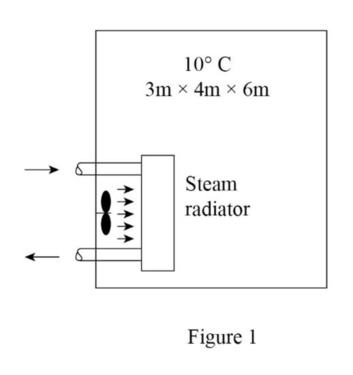
Refer the Table (A-4 through A-6), obtain the value of specific volume, specific internal energy at the initial and the final states.
At initial pressure and temperature of
The initial specific internal energy
The initial specific volume
At final pressure of
The specific volume of saturated liquid
The specific internal energy of saturated liquid
The specific volume of saturated vapour
The specific internal energy of vapour mixture
Calculate the final quality at the final state.
Calculate the final specific internal energy of a well-insulated room.
Write the expression for total mass of a water in the radiator.
Write expression for the volume of the well-insulated room.
Write the expression of mass of air in a well-insulated room.
Write the expression for amount of fan work done in 45 min.
Write the expression for the energy balance equation.
Here, the total energy entering the system is
Substitute
For well-insulated room the energy balance equation.\
Substitute
Express the change in internal energy and boundary work into the constant pressure expansion and compression in Equation (IX).
Here, the rate of heat transfer entering is
Substitute
Therefore, the well-insulated room temperature rises from
Thus, the average temperature of air in 45 min is
Want to see more full solutions like this?
Chapter 5 Solutions
Fundamentals of Thermal-Fluid Sciences
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





