
Concept explainers
(a)
The initial and the final temperature of the piston cylinder device.
(a)
Explanation of Solution
Given:
The total mass of the mixture
The initial pressure
The mass of the water
The mass of the water vapor
The final pressure of the system
The final volume of the system
Calculation:
Calculate the total initial volume of piston cylinder device.
Here, the specific volume of saturated liquid is
Calculate the total volume of the piston cylinder device at final state.
Calculate the specific volume of the piston cylinder device at final state.
Here, the mass of the saturated liquid vapour mixture of water is contained in a piston cylinder device is
Calculation:
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is saturated pressure and saturated temperature.
For initial temperature of the piston cylinder device.
Show the temperature at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (1).
|
Pressure, kPa |
Temperature, C |
| 150 kPa | 111.35 |
| 160 kPa | |
| 175 kPa | 116.04 |
Substitute the value of x and y from Table (1) in Equation (IV) to calculate the value of initial temperature
Thus, the initial temperature of the piston cylinder device is
For specific volume of saturated liquid of the piston cylinder device.
Show the specific volume of saturated liquid at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (2).
|
Pressure, kPa |
Specific volume of saturated liquid, |
| 150 kPa | 0.001053 |
| 160 kPa | |
| 175 kPa | 0.001057 |
Substitute the value of x and y from Table (2) in Equation (IV) to calculate the value of specific volume of saturated liquid
For specific volume of saturated vapour of the piston cylinder device.
Show the specific volume of saturated vapour at pressure of 150 kPa, 160 kPa, and 175 kPa as in Table (3).
|
Pressure, kPa |
Specific volume of saturated vapour, |
| 150 kPa | 1.1594 |
| 160 kPa | |
| 175 kPa | 1.0037 |
Substitute the value of x and y from Table (3) in Equation (IV) to calculate the value of specific volume of saturated vapour
Substitute
Substitute
Substitute
The unit conversion of pressure from kPa to MPa.
For temperature of the piston cylinder device at final state.
Show the temperature at specific volume of the piston cylinder device at final state at
|
specific volume of the piston cylinder device at final state, |
Temperature, |
| 600 | |
| 700 |
Substitute the value of x and y from Table (4) in Equation (IV) to calculate the value of temperature of the piston cylinder device at final state
Thus, the final temperature of the piston cylinder device is
(b)
The mass of liquid water when the piston first starts moving.
(b)
Explanation of Solution
Since,
Calculate the specific volume of the piston cylinder device at this state.
Therefore, the value of specific volume of the piston cylinder device at this state is greater than
Thus, the mass of liquid water when the piston first starts moving is
(c)
The work done during the process state 2 and 3.
(c)
Explanation of Solution
Calculate the work done in constant pressure process.
Thus, the work done during the process state 2 and 3 is
Show the P-v diagram of this process.
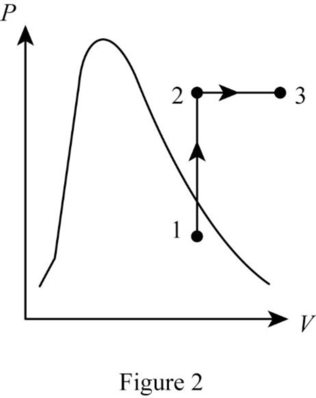
Want to see more full solutions like this?
Chapter 5 Solutions
Fundamentals of Thermal-Fluid Sciences
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





