
Concept explainers
Draw a Lewis structure and use VSEPR theory to determine the geometry of each molecule. If the molecule has more than one central atom, indicate the geometry about each of these and draw the three-dimensional structure.
Interpretation:
The Lewis structures for the given compounds are to be drawn and the geometry of each of the given molecules is to be determined and the geometry of more than one central atom is to be indicated using the VSEPR theory. The three-dimensional structures of the given molecules are to be drawn.
Concept Introduction:
According to the Lewis theory, in covalent bonds, atoms share their electrons. The steps for drawing a covalent Lewis structure are as follows:
Write the skeletal structure of the molecule.
Add the number of valence electrons of each of the atoms in the molecule to determine the total number of electrons in the molecule.
Place the electrons by dots to complete the octets of the atoms.
If the central atom has not obtained an octet, then its multiple bonds can be formed.
The VSEPR theory is helpful in predicting the shapes of molecules from their Lewis structures. The geometry of a molecule can be determined on the basis of the number of electron groups, lone pairs and bonding pairs in the molecule.
Answer to Problem 29E
Solution:
a)
Lewis structure:
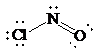
Molecular geometry is bent.
b)
Lewis structure:
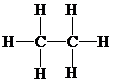
Molecular geometry is tetrahedral.
c)
Lewis structure:
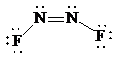
Molecular geometry is bent.
d)
Lewis structure:
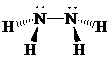
Molecular geometry is pyramidal.
Explanation of Solution
a)
The skeletal structure for the compound is as follows:
Now, the total number of electrons for the molecule is determined as follows:
Place the electrons as dots to give octet to each of the atoms in the molecule. Draw a single bond between the atoms. The central atom has not obtained an octet. Hence, nitrogen will form a double bond with oxygen to get its octet completed. Thus, the Lewis structure of
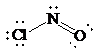
As all the atoms in the structure have obtained their octet, and thus, structure is complete. There are three electron pairs around the central atom. A double bond counts as a single electron group. Thus, there are two bonding pairs and one lone pair. Hence, according to the VSEPR theory, the molecular geometry is bent.
b)
The two carbon atoms are in the middle, each with three hydrogen atoms attached.
The skeletal structure for the compound is:
Now, the total number of electrons for the molecule is determined as follows:
Place the electrons as dots to give octet or duet to each of the atoms in the molecule. Draw a single bond between the atoms. Hence, the Lewis dot structure for the molecule will be as follows:
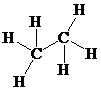
As all the hydrogen atoms in the structure have obtained their duet and both the carbon atoms have obtained their octet, thus, structure is complete. There are two central carbon atoms and so, the geometry is considered at each. There are four electron pairs around each carbon atom and no lone pair. Hence, according to the VSEPR theory, the molecular geometry is tetrahedral at each carbon atom.
c)
The two nitrogen atoms are in the centre and two fluorine atoms at the ends.
The skeletal structure for the compound is as follows:
Now, the total number of electrons for the molecule is determined as follows:
Place the electrons as dots to give octet to each of the atoms in the molecule. Draw a single bond between the atoms. Hence, the Lewis dot structure for the molecule will be as follows:
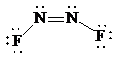
As all the atoms in the structure have obtained an octet, the structure is complete. There are two central nitrogen atoms and so, the geometry is considered at each. There are three electron groups around each nitrogen: two bonding groups and a lone pair. Hence, according to the VSEPR theory, the electron geometry is trigonal planar but the correct molecular geometry is bent.
d)
The two nitrogen atoms are in the centre and the two hydrogen atoms are attached to each nitrogen.
The skeletal structure for the compound is:
Now, the total number of electrons for the molecule is determined as follows:
Place the electrons as dots to give octet or duet to each of the atoms in the molecule. Draw a single bond between the atoms. Hence, the Lewis dot structure for the molecule will be as follows:
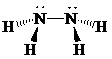
As all the hydrogen atoms in the structure have obtained their duet and the nitrogen atoms have completed their octets, the structure is complete. There are two central nitrogen atoms and so, the geometry is considered at each one. There are four electron groups around each nitrogen: three bonding groups and a lone pair. Hence, according to the VSEPR theory, the molecular geometry is trigonal pyramidal at each nitrogen atom.
Want to see more full solutions like this?
Chapter 5 Solutions
CHEMISTRY IN FOCUS (LL)-TEXT
- 1. For each molecule and ion below, indicate the total number of valence electrons. Based on that number of electrons, draw a valid Lewis structure.2. Use VSEPR theory to determine the electron arrangement and the geometry of the molecule around the central atom(s). A table of geometries is attached.3. Build the molecule using the molecular models provided in the lab. Sketch your model well enough to show the geometry. Include bond angles.4. Using your understanding of electronegativity and its trends in the Periodic Table, determine if polar bonds exist in the molecule.5. Using bond polarity and molecular shape, determine if the molecule has an overall dipole. If all the bonds are nonpolar, the molecule is nonpolar. If polar bonds are present, then consider the shape to determine if the molecule has an overall dipole.arrow_forward1. The electron pair in a H - Cl bond could be considered... a. closer to H because Hydrogen has a larger radius and thus exerts greater control over the shared electron pair b. closer to Cl because Chlorine has a higher electronegativity than Hydrogen c. closer to H because Hydrogen has a lower electronegativity than Chlorine d. an inadequate model since the bond is ionic 2. It is important to know the geometry of a molecule because the geometry _______. a. will give the Lewis structure of the molecule b. affects the physical and chemical properties of the substance c. will determine whether the molecule is ionic or covalent d. Both B, and Carrow_forwardConsider a molecule I2CO, what would be the molecular geometry? Would you predict the molecule is polar or nonpolar?arrow_forward
- Draw a lewis structure for XeF2. What is the molecular geometry around the central atom?arrow_forwardConsider the compound with the folloring lewis structure: Draw the molecule to show its correct 3 dimensional shape, then label the shape around each central atom. What are the bond angles here?arrow_forwardWhat is VSEPR theory? It is (Choose the best answer) Question 9 options: A) to predict the geometry of a molecule based on its Lewis structure B) valence electron pairs are as far away from each other as possible C) to minimize the repulsion between valence electron pairs. D) All of abovearrow_forward
- Can you draw the Lewis structure and the molecular geometry of two molecules of your choice? Write the significance or how these molecules affect our daily life.arrow_forwardH is our normal Hydrogen element E is a made up element and contains 6 valence electrons Y is a made up element and contains 7 valence electrons Calculate the number of valence electrons in HEY Using your knowledge of Lewis structures, draw the Lewis structure of HEY What is the electron geometry of HEY What is the molecular shape of HEYarrow_forwardExplain why the HOH molecule is bent, whereas the HBeH molecule is linear.arrow_forward
- Why is the geometric structure of a molecule important, especially for biological molecules?arrow_forwardClassify each of the following molecules as polar or nonpolar. You may wish to review the chapter on chemical bonding. (a) SiH4. (b) Si2H6. (c) SiCl3H. (d) SiF4. (e) SiCl2F2arrow_forwardDraw a Lewis structure and use VSEPR theory to determine the geometry of each molecule. If the molecule has more than one central atom, indicate the geometry about each of these and draw the three-dimensional structure. HCCH (two carbon atoms in the middle, each with one hydrogen atom attached) CCl4 PH3 HOOH (two oxygen atoms in the middle, each with one hydrogen atom attached)arrow_forward

 Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax





