
Concept explainers
What is the geometry around the central atom in each of the following molecular models?
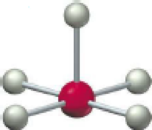
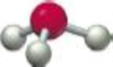


(a)
Interpretation:
The geometrical arrangement of charge clouds around the central atom in each of the given molecular models has to be given.
Concept introduction:
VSEPR model:
- Valance Shell Electron-Pair Repulsion (VSEPR) model is used to predict the shapes of the molecules by the electronic structure of its atoms.
- Electrons that are involved in bonds and in lone pairs of electrons should be thought like occupying “charge clouds” or regions of electron density.
- These region of electron density can repel one another and stay as much as possible and results to assume specific shapes.
Rules to predict the shapes of molecules by VSEPR model:
- Write electron-dot structure of the given molecule.
- Count the number of electron charge clouds surrounding the central atom.
- Determine the geometric arrangement of charge clouds surround the each atom and assume its charge clouds can be oriented in the space as far away from one to another as possible.
Explanation of Solution
According to the VSEPR model, a geometry having chemical species with 6 electron domains or electron cloud surrounding the central atom and also have 5 bonding electron pairs and 1 lone pair of electron in the chemical molecule with bond angle of
Hence, the given molecular model indicates square pyramidal geometry.
(b)
Interpretation:
The geometrical arrangement of charge clouds around the central atom in each of the given molecular models has to be given.
Concept introduction:
VSEPR model:
- Valance Shell Electron-Pair Repulsion (VSEPR) model is used to predict the shapes of the molecules by the electronic structure of its atoms.
- Electrons that are involved in bonds and in lone pairs of electrons should be thought like occupying “charge clouds” or regions of electron density.
- These region of electron density can repel one another and stay as much as possible and results to assume specific shapes.
Rules to predict the shapes of molecules by VSEPR model:
- Write electron-dot structure of the given molecule.
- Count the number of electron charge clouds surrounding the central atom.
- Determine the geometric arrangement of charge clouds surround the each atom and assume its charge clouds can be oriented in the space as far away from one to another as possible.
Explanation of Solution
According to the VSEPR model, a geometry having chemical species with 4 electron domains or electron cloud surrounding the central atom and also have 3 bonding electron pairs and 1 lone pair of electron in the chemical molecule with bond angle of
Hence, the given molecular model indicates trigonal pyramidal geometry.
(c)
Interpretation:
The geometrical arrangement of charge clouds around the central atom in each of the given molecular models has to be given.
Concept introduction:
VSEPR model:
- Valance Shell Electron-Pair Repulsion (VSEPR) model is used to predict the shapes of the molecules by the electronic structure of its atoms.
- Electrons that are involved in bonds and in lone pairs of electrons should be thought like occupying “charge clouds” or regions of electron density.
- These region of electron density can repel one another and stay as much as possible and results to assume specific shapes.
Rules to predict the shapes of molecules by VSEPR model:
- Write electron-dot structure of the given molecule.
- Count the number of electron charge clouds surrounding the central atom.
- Determine the geometric arrangement of charge clouds surround the each atom and assume its charge clouds can be oriented in the space as far away from one to another as possible.
Explanation of Solution
According to the VSEPR model, a geometry having chemical species with 6 electron domains or electron cloud surrounding the central atom and also have 4 bonding electron pairs and 2 lone pairs of electron in the chemical molecule with bond angle of
Hence, the given molecular model indicates square planar geometry.
(d)
Interpretation:
The geometrical arrangement of charge clouds around the central atom in each of the given molecular models has to be given.
Concept introduction:
VSEPR model:
- Valance Shell Electron-Pair Repulsion (VSEPR) model is used to predict the shapes of the molecules by the electronic structure of its atoms.
- Electrons that are involved in bonds and in lone pairs of electrons should be thought like occupying “charge clouds” or regions of electron density.
- These region of electron density can repel one another and stay as much as possible and results to assume specific shapes.
Rules to predict the shapes of molecules by VSEPR model:
- Write electron-dot structure of the given molecule.
- Count the number of electron charge clouds surrounding the central atom.
- Determine the geometric arrangement of charge clouds surround the each atom and assume its charge clouds can be oriented in the space as far away from one to another as possible.
Explanation of Solution
According to the VSEPR model, a geometry having chemical species with 3 electron domains or electron cloud surrounding the central atom and also have 3 bonding electron pairs in the chemical molecule with bond angle of
Hence, the given molecular model indicates trigonal planar geometry.
Want to see more full solutions like this?
Chapter 5 Solutions
General Chemistry: Atoms First-Solution Manual
- Use the VSEPR model to predict the bond angles around each central atom in the following Lewis structures (benzene rings are frequently pictured as hexagons, without the letter for the carbon atom at each vertex). Note that the drawings do not necessarily depict the bond angles correctly.arrow_forward• explain how hybridization reconciles observed molecular shapes with the orbital overlap model.arrow_forwardFor each of the following molecules, state the bond angle (or bond angles, as appropriate) that you would expect to see on the central atom based on the simple VSEPR model. Would you expect the actual bond angles to be greater or less than this? a CCl4 b SCl2 c COCl2 d AsH3arrow_forward
- • identify sigma and pi bonds in a molecule and explain the difference between them.arrow_forwardIndicate whether each of the following molecules is polar or nonpolar. The molecular geometry is given in parentheses. a. NF3 (trigonal pyramidal with N at the apex) b. NF2Cl (trigonal pyramidal with N at the apex) c. CS2 (linear with C in the center position) d. CHCl3 (tetrahedral with C in the center position)arrow_forwardIndicate which molecules are polar and which are nonpolar. (a) SeO2 (b) N2O (N is the central atom) (c) SCl4arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





