
Concept explainers
The amino acid
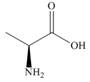
(S)-alanine
a. What is the melting point of
b. How does the melting point of a racemic mixture of
c. What is the specific rotation of
d. What is the optical rotation of a racemic mixture of
e. Label each of the following as optically active or inactive: a solution of pure
(a)
Interpretation: The melting point of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The melting point of
Explanation of Solution
The given melting point of
The melting point of
(b)
Interpretation: The melting point of a racemic mixture of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The melting point of a racemic mixture of
Explanation of Solution
The given melting point of
Therefore, the melting point of a racemic mixture of
The melting point of a racemic mixture of
(c)
Interpretation: The specific rotation of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The specific rotation of
Explanation of Solution
The specific rotation of
Thus, the specific rotation of
The specific rotation of
(d)
Interpretation: The optical rotation of a racemic mixture of
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The optical rotation of a racemic mixture of
Explanation of Solution
The specific rotation of
The specific rotation of
The
Thus, the optical rotation of a racemic mixture of
The optical rotation of a racemic mixture of
(e)
Interpretation: The solutions of pure
Concept introduction: Enantiomers are the stereoisomers that have exactly opposite
Answer to Problem 5.28P
The pure
Explanation of Solution
The pure
A racemic mixture is obtained when
A
Thus, the pure
The pure
Want to see more full solutions like this?
Chapter 5 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
Additional Science Textbook Solutions
General Chemistry: Atoms First
Chemistry: Atoms First
Elementary Principles of Chemical Processes, Binder Ready Version
Organic Chemistry As a Second Language: Second Semester Topics
Chemistry
EBK INTRODUCTION TO CHEMISTRY
- Esterase is an enzyme that catalyzes the hydrolysis of esters. It hydrolyzes esters of L-amino acids more rapidly than esters of d-amino acids. How can this enzyme be used to separate a racemic mixture of amino acids?arrow_forwardIn the structures of T—A and C—G base pairs, there are three amino groups specifically labeled as “sp2 hybridized and planar”. What is the primary difference between these structures and that of aniline that lead to their planarity? 1. In contrast to aniline, the amino groups on the DNA bases are necessary to make the heterocyclic rings aromatic. 2. In contrast to aniline, the contributing structures that delocalize the nitrogen lone pairs onto the rings creates partial negative charges on electronegative atoms. 3. In contrast to aniline, the hydrogen bond accepting ability of the lone pairs on the -NH2 groups of the DNA bases is better when these amino groups are sp2 hybridized. 4. Both 2 and 3.arrow_forwardA sample of a saccharide turns dark blue with subjected to the starch iodine test. What does this result indicate about the saccharide?arrow_forward
- (i) Why do actinoids show wide range of oxidation states? (ii) Why is actinoid contraction greater than lanthanoid contraction?arrow_forwardDefine tautomerism and use one of the structures above to illustrate your explanationarrow_forwardDraw the structure of leu-enkephalin, a pentapeptide that acts as an analgesic and opiate, and has the following sequence: Tyr–Gly–Gly–Phe–Leu. (The structure of a related peptide, met-enkephalin, appeared in Section 22.6B.)arrow_forward
- Is fluoxetine chiral? If so, which of the possible stereoisomers are formed in this synthesis?arrow_forwardAswer all parts of the question. A freshly prepared solution of αα ‑D‑glucose shows a specific rotation of +112°.+112°. Over time, the rotation of the solution gradually decreases and reaches an equilibrium value corresponding to [?]25 °CD=+52.5°.[α]D25 °C=+52.5°. In contrast, a freshly prepared solution of ?β‑D‑glucose has a specific rotation of +19°.+19°. The rotation of this solution increases over time to the same equilibrium value as that shown by the ?α anomer. A solution of one enantiomer of a given monosaccharide rotates plane‑polarized light to the left (counterclockwise) and is the levorotatory isomer, designated (−). The other enantiomer rotates plane‑polarized light to the same extent but to the right (clockwise) and is the dextrorotatory isomer, designated (+). An equimolar mixture of the (+) and (−) forms does not rotate plane‑polarized light. The optical rotation, the number of degrees by which plane‑polarized light rotates on passage through a given path length of a…arrow_forwardWhat is the structure and components/composition of Cellulose in Fischer and Haworth form? Please explainarrow_forward
- Why is it impossible to have only one enantiomer of thalidomide under biological conditions?arrow_forwardIs proparacaine chiral? If so, how many of the possible stereoisomers are formed in this synthesis?arrow_forwardConsider the following tetrapeptide, written in a sequence according to normal convention, from left to right: Residue 1 is acidic; Residue 2 is cysteine (an amino acid containing a –CH2SH R-group); Residue 3 is proline (a large hydrophobic amino acid); Residue 4 is basic. Of the following four general statements, which one is TRUE regarding this tetrapeptide? This tetrapeptide does not have the ability to form a disulfide linkage with an identical tetrapeptide. Residue 2 belongs to the amino acid category known as "basic." 3 of these 4 responses are correct This tetrapeptide could be part of an alpha-helix, since the large size of the proline would not have any impact on the consistency of the spiral shape. The acidic residue (Residue #1) is at the amino terminus of this tetrapeptide.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


