
5.53.20 Chemicals are stored in a laboratory with volume V(m3). As a consequence of poor laboratory practices, a hazardous species, A, enters the room air (from inside the room) at a constant rate 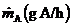 The room is ventilated with clean air ?owing at a constant rate
The room is ventilated with clean air ?owing at a constant rate 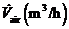 The average concentration of A in the room air builds up until it reaches a steady-state value
The average concentration of A in the room air builds up until it reaches a steady-state value 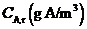
(a) List at least four situations that could lead to A getting into the room air.
(b) Assume that the A is perfectly mixed with the room air and derive the formula
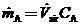
(c) The assumption of perfect mixing is never justi?ed when the enclosed space is a room (as opposed to, say, a stirred reactor). In practice, the concentration of A varies from one point in the room to another: it is relatively high near the point where A enters the room air and relatively low in regions far from that point, including the ventilator outlet duct. If we say that 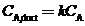 where
where 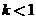 is a nonideal mixing factor (generally between 0.1 and 0.5, with the lowest value corresponding to the poorest mixing). then the equation of Part (b) becomes
is a nonideal mixing factor (generally between 0.1 and 0.5, with the lowest value corresponding to the poorest mixing). then the equation of Part (b) becomes
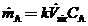
Use this equation and the ideal-gas equation of state to derive the following expression for the average mole fraction of A in the room air:
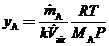
where MA is the molecular weight of A.
(b) The permissible exposure level (PEL) for styrene (M = 104.14) de?ned by the U.S. Occupational Safety and Health Administration is 50 ppm (molar basis).21 An open storage tank in a polymerization laboratory contains styrene. The evaporation rate from this tank is estimated to be 9.0 g/h. Room temperature is 20°C. Assuming that the laboratory air is reasonably well mixed (so that k = 0.5), calculate the minimum ventilation rate (m3/h) required to keep the average styrene concentration at or below the PEL. Then give several reasons why working in the laboratory might still be hazardous if the calculated minimum ventilation rate is used.
(e) Would the hazard level in the situation described in Part (d) increase or decrease if the temperature in the room were to increase? (Increase, decrease, no way to tell.) Explain your answer, citing at least two effects of temperature in your explanation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Concepts Of Programming Languages
C How to Program (8th Edition)
Starting Out with C++ from Control Structures to Objects (9th Edition)
Automotive Technology: Principles, Diagnosis, And Service (6th Edition) (halderman Automotive Series)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





