
Concept explainers
(a)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
The
![]()
Figure 1
The electron dot structure of
(b)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
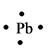
Explanation of Solution
Lead belongs to the group
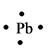
Figure 2
The electron dot structure of
(c)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Selenium belongs to the group
![]()
Figure 3
The electron dot structure of
(d)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Neon belongs to the group
![]()
Figure 4
The electron dot structure of
(e)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Cesium belongs to the group
![]()
Figure 5
The electron dot structure of
(f)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
![]()
Explanation of Solution
Gallium belongs to the group
![]()
Figure 6
The electron dot structure of
(g)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
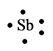
Explanation of Solution
Antimony belongs to the group
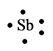
Figure 7
The electron dot structure of
(h)
Interpretation:
The electron dot structure for the atom of
Concept introduction:
The electron dot formula is used for the identification of valence electrons present in an atom. It shows the symbol of an atom with its valence electron around it. It is also called as Lewis dot structure. The valence electrons around an atom are shown by dots. One dot represents one valence electron.
The rules to draw electron dot formula are given below.
• First, the symbol of an element is written.
• With the help of group number, the number of valence electrons is determined.
• The valance electrons are drawn around the atom by dots.
Answer to Problem 62E
The electron dot structure for the atom of
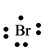
Explanation of Solution
Bromine belongs to the group
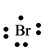
Figure 8
The electron dot structure of
Want to see more full solutions like this?
Chapter 5 Solutions
INTRODUCTORY CHEMISTRY W/ACCESS
- A main group element with the valence electron configuration 2s1 is in periodic group __________.It forms a monatomic ion with a charge of ___________.arrow_forwarda. Explain why a positive ion is always smaller than itsparent atom.b. Explain why a fluoride ion is commonly found in naturebut a fluorine atom is notarrow_forwardWhich order of ionization energy level should the following go in? Oxygen, manganese, silicon, barium, nickelarrow_forward
- Completely the following sentence or (illustrate the idea) Based on the most common ion of Lithium, it’s more likely to lose/or gain electrons. From this I can infer that the attractive force between the nucleus and its valence electrons is relatively (strong /weak )arrow_forwardWhy is the ion not found in nature?arrow_forward• use electron configurations to explain why metals tend to form cations whereas nonmetals tend to form anions.arrow_forward
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





