
The chapter sections to review are shown in parentheses at the end of each problem.
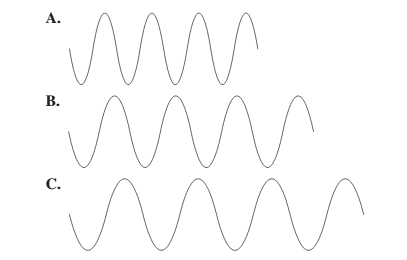
Use the following diagram tor problems 5.77 and 5.78:
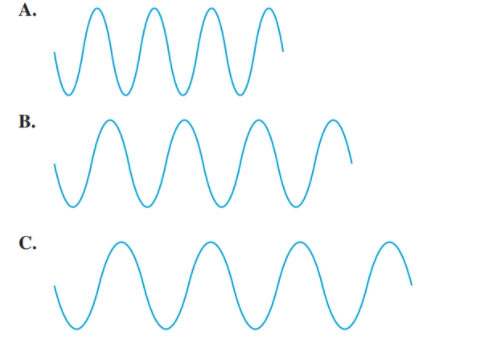
Select diagram A, B, or C that (5.1)
a. has the longest wavelength
b. has the shortest wavelength
c. has the highest frequency
d. has the lowest frequency
(a)
Interpretation:
The diagram having the longest wavelength should be selected.
Concept Introduction:The distance between two successive identical parts of the wave (from successive trough to trough or crest to crest) is said to be the wavelength.
Answer to Problem 77UTC
C.
Explanation of Solution
Given:
Larger the distance between the successive crest to crest or trough to trough, larger is the wavelength.
Among the given waves, the distance between successive crest to crest of wave C is largest so, the wavelength of wave C is longest.
(b)
Interpretation:
The diagram having the shortest wavelength should be selected.
Concept Introduction: The distance between two successive identical parts of the wave (from successive trough to trough or crest to crest) is said to be the wavelength.
Answer to Problem 77UTC
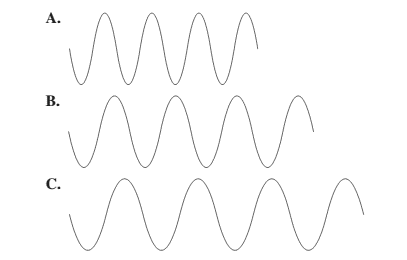
A.
Explanation of Solution
Given:
Smaller the distance between the successive crest to crest or trough to trough, smaller is the wavelength.
Among the given waves, the distance between successive crest to crest of wave A is smallest so, the wavelength of wave A is shortest.
(c)
Interpretation:
The diagram having the highest frequency should be selected.
Concept Introduction: The relationship between wavelength and frequency is given as:
Where
Answer to Problem 77UTC
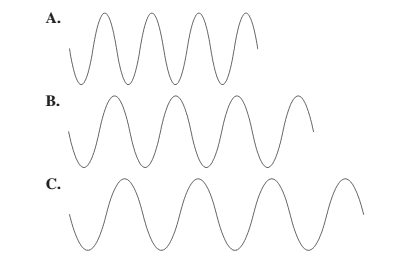
A.
Explanation of Solution
Given:
There is a relationship between wavelength and frequency that is they are inversely proportional to each other. Higher the value of wavelength, lower will be the frequency and vice-versa.
Thus, wave A will have highest frequency.
(d)
Interpretation:
The diagram having the lowest frequency should be selected.
Concept Introduction: The relationship between wavelength and frequency is given as:
Where
Answer to Problem 77UTC
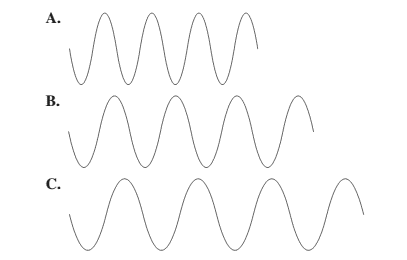
C.
Explanation of Solution
Given:
There is a relationship between wavelength and frequency that is they are inversely proportional to each other. Lower the value of wavelength, higher will be the frequency and vice-versa.
Thus, wave C will have lowest frequency.
Want to see more full solutions like this?
Chapter 5 Solutions
Basic Chemistry Plus Mastering Chemistry With Pearson Etext -- Access Card Package (6th Edition)
- The energy required to split apart dioxygen according to the equation is 8.17 × 10-19 J/molecule. O2(g) → 2O(g) Calculate the frequency (Hz) of the photon that can dissociate dioxygen. Express answer in scientific notation.arrow_forwardCan you plz help me, I am reviewing over a PowerPoint and I don’t know how they got 0.84 for C can you plz explain thankyou so mucharrow_forwardWhat is the velocity of electrons emitted from K metal (threshold energy = 2.25eV) using incident radiation of 6.2eV. Input only answer for velocity in m/sarrow_forward
- A light photon with a wavelength no greater than 406 × 10-9 m is required to split apart hydrogen iodide according to the equation: HI(g) → H(g) + I(g) Calculate the energy (kJ) required to break apart a mole of hydrogen iodide. Express answer in scientific notation.arrow_forward3) Electrons are filled in various sub-shells from higher energy to lower energy. Select one: True Falsearrow_forwardThe nucleus of an atom contains:A. electrons and protons only.B. protons only.C. electrons, protons, and neutrons.D. protons and neutrons only.Reset SelectionMark for Review What’s This?arrow_forward
- (MULTIPLE CHOICE) This dot structure represents which of the following answers: - [Ne] 3s^2 3p^4 - S^2- - [Ne] 3s^2 3p^6 - S^2+ - Sarrow_forwardA light photon with a frequency of at least 0.234 × 1016 Hz is required to split apart dinitrogen according to the equation: N2(g) → 2N(g) Calculate the energy (kJ) required to break apart a mole of dinitrogen. Express answer in scientific notation.arrow_forwardfor D, how did we get 0.693?arrow_forward
- 60 When a photon of light with a wavelength of 359 nm is shone on a metal surface, an electron is ejected with a kinetic energy of 1.28×10-19 J. What is the binding energy of the metal in kJ/mol? Express your answer to three significant figures. Φ = Answer kJ/molarrow_forwardQ-nirali The chemical reaction of Octane combustion is shown below: CgH18 + 20(O₂ + 3.76N₂) Find value of z?arrow_forwardHow energetic a photon is required to strip an electron away from a completely relaxed Hydrogen atom? The wavelength of the photon is 92 nm. Answer in scientific notation.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





