
Chemistry: Structure and Properties Plus Mastering Chemistry with Pearson eText -- Access Card Package (2nd Edition) (New Chemistry Titles from Niva Tro)
2nd Edition
ISBN: 9780134436524
Author: Nivaldo J. Tro
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 5, Problem 90E
Free radicals are important in many environmentally significant reactions. For example, photochemical smog—smog that results
from the action of sunlight on air pollutants—forms in part by these two steps:
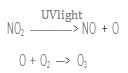
The product of this reaction, ozone, is a pollutant in the lower atmosphere. (Upper atmospheric ozone is a natural part of the atmosphere that protects life on Earth from ultraviolet light.) Ozone is an eye and lung irritant and also accelerates the weathering of rubber products. Rewrite the above reactions using the Lewis structure of each reactant and product. Identify the free radicals.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Phosgene, a substance used in poisonous gas warfare during World War I, is so named because it was first prepared by the action of sunlight on a mixture of carbon monoxide and chlorine gases. Its name comes from the Greek words phos (light) and genes (born of). Phosgene has the following elemental composition: 12.14% C, 16.17% O, and 71.69% Cl by mass. Its molar mass is 98.9 g/mol.
(d) Using average bond enthalpies, estimate H for the formation of gaseous phosgene from CO(g) and Cl2(g).
Draw three resonance structures for N3-. This species has its three atoms bonded sequentially in the following fashion: N-N-N. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure.In the most important resonance structure of N3- :
a) The leftmost bond (between N and N) is a single bond.
b) The rightmost bond (between N and N) is a single bond.
c) The formal charge on the leftmost (N) atom is -1.
d) The number of nonbonding pairs (lone pairs) of electrons on the leftmost (N) atom is 4.
e) The number of nonbonding (lone) pairs of electrons on the rightmost (N) atom is 4.
Draw three resonance structures for N3-. This species has its three atoms bonded sequentially in the following fashion: N-N-N. Draw your resonance structures so that the atoms in them are bonded together in this order. Select the most important resonance structure for this species based on the formal charges on the atoms of the three resonance structures you have drawn. Now select the statement from the multiple choices which is true about this most important resonance structure.In the most important resonance structure of N3- :
a) The leftmost bond (between N and N) is a single bond.b) The rightmost bond (between N and N) is a single bond.c) The formal charge on the leftmost (N) atom is -1.d) The number of nonbonding pairs (lone pairs) of electrons on the leftmost (N) atom is 4.e) The number of nonbonding (lone) pairs of electrons on the rightmost (N) atom is 4.
Chapter 5 Solutions
Chemistry: Structure and Properties Plus Mastering Chemistry with Pearson eText -- Access Card Package (2nd Edition) (New Chemistry Titles from Niva Tro)
Ch. 5 - What is electronegativity? What are the periodic...Ch. 5 - Explain the difference between a pure covalent...Ch. 5 - What is meant by the percent ionic character of a...Ch. 5 - Prob. 4ECh. 5 - What is the magnitude of the dipole moment formed...Ch. 5 - What is the basic procedure for writing a covalent...Ch. 5 - How do you determine the number of electrons that...Ch. 5 - What are resonance structures? What is a resonance...Ch. 5 - Prob. 9ECh. 5 - Prob. 10E
Ch. 5 - Prob. 11ECh. 5 - Prob. 12ECh. 5 - What is bond energy?Ch. 5 - Give some examples of some typical bond lengths....Ch. 5 - Why is molecular geometry important? Cite some...Ch. 5 - According to VSEPR theory, what determines the...Ch. 5 - Name and draw the five basic electron geometries,...Ch. 5 - Explain the difference between electron geometry...Ch. 5 - List the correct electron and molecular geometries...Ch. 5 - How do you apply VSEPR theory to predict the shape...Ch. 5 - How do you determine if a molecule is polar?Ch. 5 - Why is polarity a key connection between the...Ch. 5 - Prob. 23ECh. 5 - Determine if a bond between each pair of atoms...Ch. 5 - Prob. 25ECh. 5 - Draw the Lewis structure for BrF with an arrow...Ch. 5 - Prob. 27ECh. 5 - Write the Lewis structure for each molecule. NF3...Ch. 5 - Prob. 29ECh. 5 - Write the Lewis structure for each molecule. CH2O...Ch. 5 - Prob. 31ECh. 5 - Prob. 32ECh. 5 - Write the Lewis structure for each molecule or ion...Ch. 5 - Prob. 34ECh. 5 - Write a Lewis structure that obeys the octet rule...Ch. 5 - Prob. 36ECh. 5 - Use formal charge to determine which Lewis...Ch. 5 - Prob. 38ECh. 5 - How important is this resonance structure to the...Ch. 5 - Prob. 40ECh. 5 - Prob. 41ECh. 5 - Prob. 42ECh. 5 - Determine the formal charges of the atoms shown in...Ch. 5 - Prob. 44ECh. 5 - Prob. 45ECh. 5 - Write the Lewis structure for each molecule (octet...Ch. 5 - Prob. 47ECh. 5 - Write Lewis structures for each molecule or ion....Ch. 5 - Prob. 49ECh. 5 - Write Lewis structures for each molecule or ion....Ch. 5 - List these compounds in order of increasing...Ch. 5 - Which of these compounds has the stronger...Ch. 5 - A molecule with the formula AB3 has a trigonal...Ch. 5 - A molecule with the formula AB3 has a trigonal...Ch. 5 - For each molecular geometry shown here, list the...Ch. 5 - For each molecular geometry shown here, list the...Ch. 5 - Determine the electron geometry, molecular...Ch. 5 - Determine the electron geometry, molecular...Ch. 5 - Which species has the smaller bond angle, H3O+ or...Ch. 5 - Which species has the smaller bond angle; C1O4- or...Ch. 5 - Determine the molecular geometry and draw each...Ch. 5 - Determine the molecular geometry and draw each...Ch. 5 - Determine the molecular geometry about each...Ch. 5 - Prob. 64ECh. 5 - Prob. 65ECh. 5 - Prob. 66ECh. 5 - Prob. 67ECh. 5 - Determine the geometry about each interior atom in...Ch. 5 - Explain why CO2 and CCl4 are both nonpolar even...Ch. 5 - CH3F is a polar molecule, even though the...Ch. 5 - Determine whether each molecule in Exercise 57 is...Ch. 5 - Prob. 72ECh. 5 - Determine whether each molecule or ion is polar or...Ch. 5 - Determine whether each molecule is polar or...Ch. 5 - Each compound contains both ionic and covalent...Ch. 5 - Prob. 76ECh. 5 - Carbon ring structures are common in organic...Ch. 5 - Prob. 78ECh. 5 - Prob. 79ECh. 5 - Diazomethane is a highly poisonous, explosive...Ch. 5 - Prob. 81ECh. 5 - Phosgene (Cl2CO) is a poisonous gas that was used...Ch. 5 - The cyanate ion (OCN-) and the fulminate ion...Ch. 5 - Prob. 84ECh. 5 - Prob. 85ECh. 5 - Prob. 86ECh. 5 - Prob. 87ECh. 5 - Prob. 88ECh. 5 - Prob. 89ECh. 5 - Free radicals are important in many...Ch. 5 - A compound composed of only carbon and hydrogen is...Ch. 5 - A compound composed of only carbon and chlorine is...Ch. 5 - Prob. 93ECh. 5 - The genetic code is based on four different bases...Ch. 5 - Prob. 95ECh. 5 - Prob. 96ECh. 5 - Prob. 97ECh. 5 - A 0.167-g sample of an unknown compound contains...Ch. 5 - Use the dipole moments of HF and HCI (given at the...Ch. 5 - One form of phosphorus exists as P4 molecules....Ch. 5 - A compound has the formula C8H8 and does not...Ch. 5 - Draw the Lewis structure for acetamide (CH3CONH2),...Ch. 5 - Prob. 103ECh. 5 - In the very first chapter of this book, we...Ch. 5 - Which statement best captures the fundamental idea...Ch. 5 - Prob. 106ECh. 5 - Have each member of your group represent an atom...Ch. 5 - Prob. 108ECh. 5 - Prob. 109ECh. 5 - Prob. 110ECh. 5 - Pass a piece of paper around the group and ask...Ch. 5 - Prob. 112ECh. 5 - At least two different numbers of electron groups...Ch. 5 - Prob. 114ECh. 5 - The VSEPR model is useful in predicting bond...Ch. 5 - Which set of elements is arranged in order of...Ch. 5 - Prob. 2SAQCh. 5 - Which pair of atoms forms the most polar bond? C...Ch. 5 - Which pair of atoms forms a nonpolar covalent...Ch. 5 - Prob. 5SAQCh. 5 - Prob. 6SAQCh. 5 - Prob. 7SAQCh. 5 - Prob. 8SAQCh. 5 - Prob. 9SAQCh. 5 - Prob. 10SAQCh. 5 - Prob. 11SAQCh. 5 - Predict the relative bond angles in BF3 and SO2Ch. 5 - Predict the molecular geometry about N in the...Ch. 5 - Which molecule is polar?
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A paper published in the research Journal Science in 2007 (S. Vallina and R. Simo, Science, Vol. 315, p. 506, January 26, 2007) reported studies of dimethylsulfide (DMS), an important green-house gas that is released by marine phytoplankton. This gas represents the largest natural source of atmospheric sulfur and a major precursor of hygroscopic (i.e., cloud-forming) particles in clean air over the remote oceans, thereby acting to reduce the amount of solar radiation that crosses the atmosphere and is absorbed by the ocean. (a) Sketch the Lewis structure of dimethylsulfide, CH3SCH3, and list the bond angles in the molecule. (b) Use electronegativities to decide where the positive and negative charges lie in the molecule. Is the molecule polar? (c) The mean seawater concentration of DMS in the ocean in the region between 15 north latitude and 15 south latitude is 2.7 nM (nanomolar). How many molecules of DMS are present in 1.0 m3 of seawater?arrow_forwardChloromethane has the Lewis structure _______________________________ The carbon atom is sharing 4 electron pairs. In each shared pair the carbon atom “owns” 1 electron. The number of electrons that “belong” to carbon is ___. Carbon, being a Group ___ element would have 4 , outer shell electrons in the unbonded, neutral state. Therefore, the carbon atom in chloromethane has a formal charge of zero.arrow_forwarda Carbonyl fluoride, COF2, is an extremely poisonous gas used in organofluorine synthesis. Give the valence bond description of the carbonyl fluoride molecule. (Both fluorine atoms are attached to the carbon atom.) b Nitrogen, N2, makes up about 80% of the earths atmosphere. Give the valence bond description of this molecule.arrow_forward
- Methylcyanoacrylate is the active ingredient in super glues. Its Lewis structure is In this molecule, which is the (a) weakest carbon-containing bond? (b) strongest carbon-containing bond? (c) most polar bond?arrow_forwardConsider hypothetical elements X2 and Y2. Suppose the enthalpy of formation of the compound XY is – 84 kJ/mol, the bond energy for X2 is 105 kJ/mol, and the bond energy for Y2 is 58 kJ/mol. A)Estimate the XY bond energy, Ed, in units of kJ/mol. B)If the dissociation of a X-Y molecule were accomplished by the absorption of a single photon whose energy was exactly the quantity required, what would be its wavelength in nm? C)Would a green-light photon (λgreen=550 nm) be able to break the above X-Y bond? Explain.arrow_forwardIf the formation of chemical bonds always releases energy, why don’t all elements form dozens of bonds to each atom? When fossil fuels containing sulfur are burned in power plants to generate electricity, large amounts of sulfur dioxide (SO2) are formed and released into the atmosphere, where some of it eventually forms the acid in acid rain. If the structure of SO2 consists of a central sulfur atom bonded to both oxygen atoms, draw two resonance structures for sulfur dioxide.arrow_forward
- The formula: SOF2 what is the 3D Lewis structure with bond angles and all dipole moments?what is the least electronegative element? What is the number of electron regions surrounding the central atom ? is it octet exceeded? what is the genereral formula? Is it polar or non polar molecule? what is the molecular geometry? what is the electron pair geometry?arrow_forwardDraw the Lewis structure of SO₂ (by following the octet rule on all atoms) and then determine the ideal bonding angle(s) of the central atom. +arrow_forwardThe formula for nitryl chloride is CINO2 (in which N is the central atom). a.Draw the Lewis structure for the molecule, including all resonance structures. b.What is the N-O bond order? c.Describe the electron-pair and molecular geometries and give values for all bond angles. d.What is the most polar bond in the molecule? Is the molecule polar? e.The computer program used to calculate electrostatic potential surfaces gave the following charges on atoms in the molecule: A =-0.03, B = -0.26, and C = +0.56. Identify the atoms A, B, and C. Are these calculated charges in accord with your predictions?arrow_forward
- The formula: BrCN what is the 3D Lewis structure with bond angles and all dipole moments? what is the least electronegative element? What is the number of electron regions surrounding the central atom ? is it octet exceeded? what is the genereral formula? Is it polar or non polar molecule? what is the molecular geometry? what is the electron pair geometry?arrow_forwardWhat is the bond energy for this chemical equation? C2H2 + 2.5O2 yields 2CO2 + H2Oarrow_forwardThe best lewis dot structure of the NO2 molecule has A. an unpaired electron on the nitrogen atomB. an unpaired electron on the oxygen atom C. A triple bond between N and one of the oxygens D. An expanded octet on Narrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

INTRODUCTION TO MOLECULAR QUANTUM MECHANICS -Valence bond theory - 1; Author: AGK Chemistry;https://www.youtube.com/watch?v=U8kPBPqDIwM;License: Standard YouTube License, CC-BY