
(a)
Interpretation: The electron configuration for the boron atom needs to be written and the number of unpaired electrons present in it needs to be determined.
Concept Introduction: The electron configuration explains the electron distribution in atomic orbitals. There is a standard notation to write an electron configuration. The atomic shell is written in a sequence with the number of electrons in superscript.
(a)
Answer to Problem 9SP
Explanation of Solution
The given atom is boron. It belongs to group 13 with
To determine the number of unpaired electrons, the atomic orbitals can be drawn as follows:
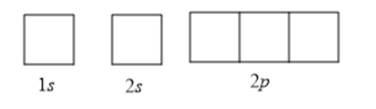
Now according to Hund’s rule, the filling of electrons in orbitals takes place in such a way that every orbital is singly occupied before any orbital is doubly occupied by electrons. Also, according to the Pauli-exclusion principle, two electrons in the same orbital must have opposite spins.
Now, electrons are filled as follows:
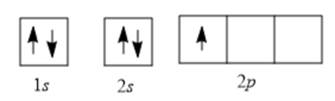
There is 1 unpaired electron in the 2p orbital; thus, the number of unpaired electrons will be 1.
(b)
Interpretation: The electron configuration for the silicon atom needs to be written and the number of unpaired electrons present in it needs to be determined.
Concept Introduction: The electron configuration explains the electron distribution in atomic orbitals. There is a standard notation to write an electron configuration. The atomic shell is written in a sequence with the number of electrons in superscript.
(b)
Answer to Problem 9SP
Explanation of Solution
The given atom is silicon. It belongs to group 14 with atomic number 14. Since the maximum electrons hold by s and p orbitals are 2 and 6 respectively. The electronic configuration is represented as follows:
To determine the number of unpaired electrons, the atomic orbitals can be drawn as follows:
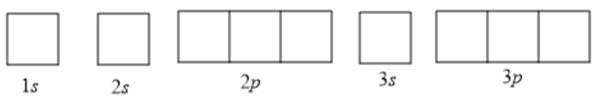
Now according to Hund’s rule, the filling of electrons in orbitals takes place in such a way that every orbital is singly occupied before any orbital is doubly occupied by electrons. Also, according to the Pauli-exclusion principle, two electrons in the same orbital must have opposite spins.
Now, electrons are filled as follows:
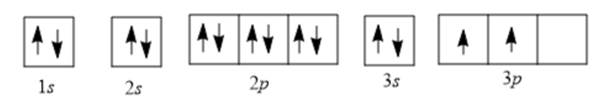
There are 2 unpaired electrons in the 3p orbital; thus, the number of unpaired electrons will be 2.
(c)
Interpretation: The electron configuration for the sulfur atom needs to be written and the number of unpaired electrons present in it needs to be determined.
Concept Introduction: The electron configuration explains the electron distribution in atomic orbitals. There is a standard notation to write an electron configuration. The atomic shell is written in a sequence with the number of electrons in superscript.
(c)
Answer to Problem 9SP
Explanation of Solution
The given atom is sulfur. It belongs to group 16 with the atomic number 16. Since the maximum electrons hold by s and p orbitals are 2 and 6 respectively. The electronic configuration is represented as follows:
To determine the number of unpaired electrons, the atomic orbitals can be drawn as follows:
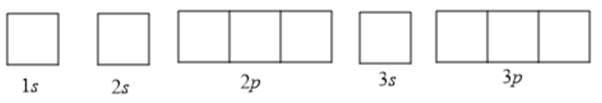
Now according to Hund’s rule, the filling of electrons in orbitals takes place in such a way that every orbital is singly occupied before any orbital is doubly occupied by electrons. Also, according to the Pauli-exclusion principle, two electrons in the same orbital must have opposite spins.
Now, electrons are filled as follows:
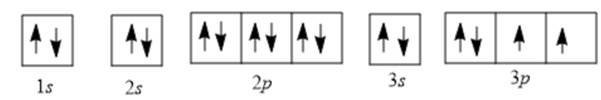
There are 2 unpaired electrons in the 3p orbital; thus, the number of unpaired electrons will be 2.
Chapter 5 Solutions
Chemistry 2012 Student Edition (hard Cover) Grade 11
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





