
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 17PS
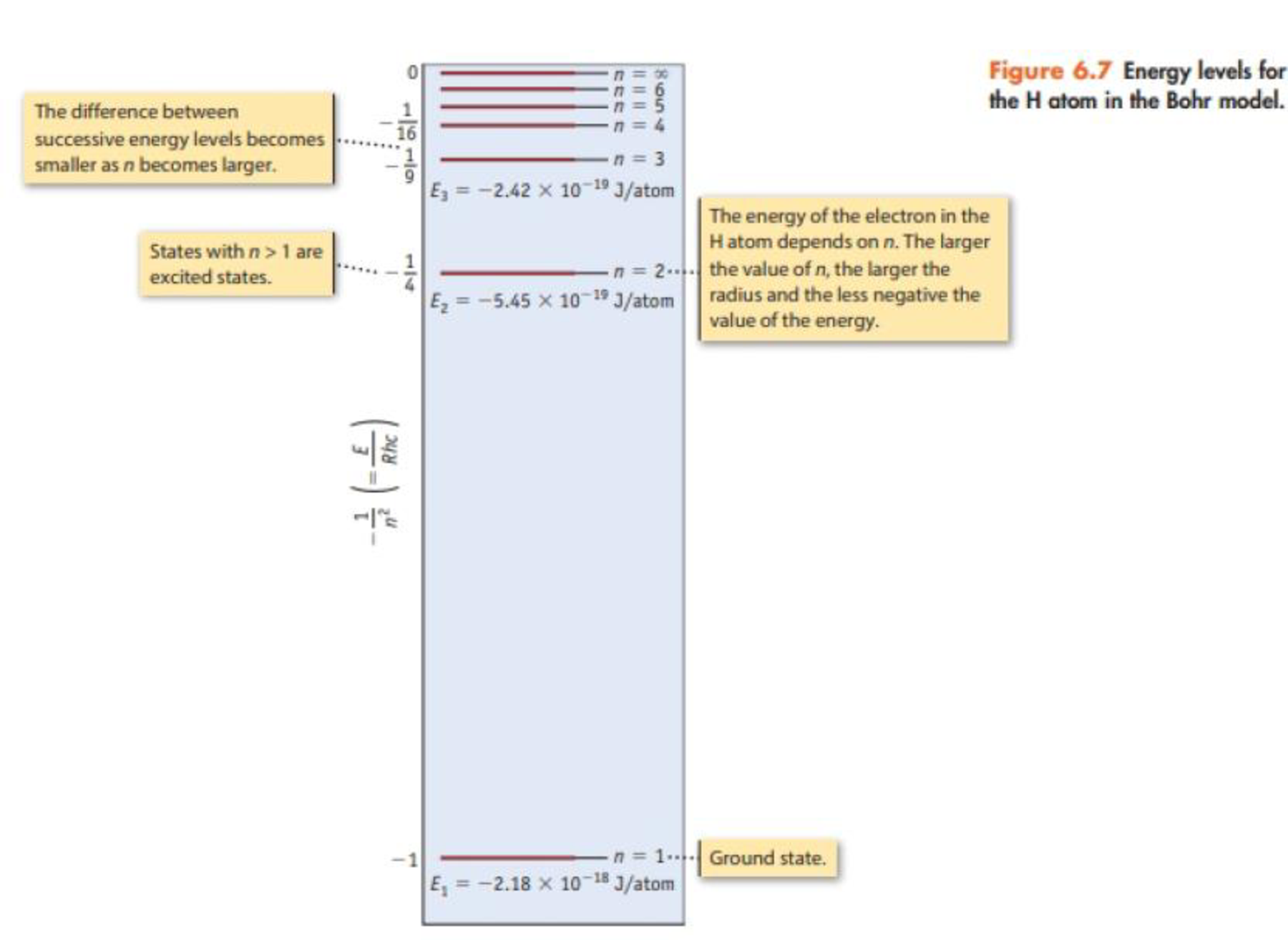
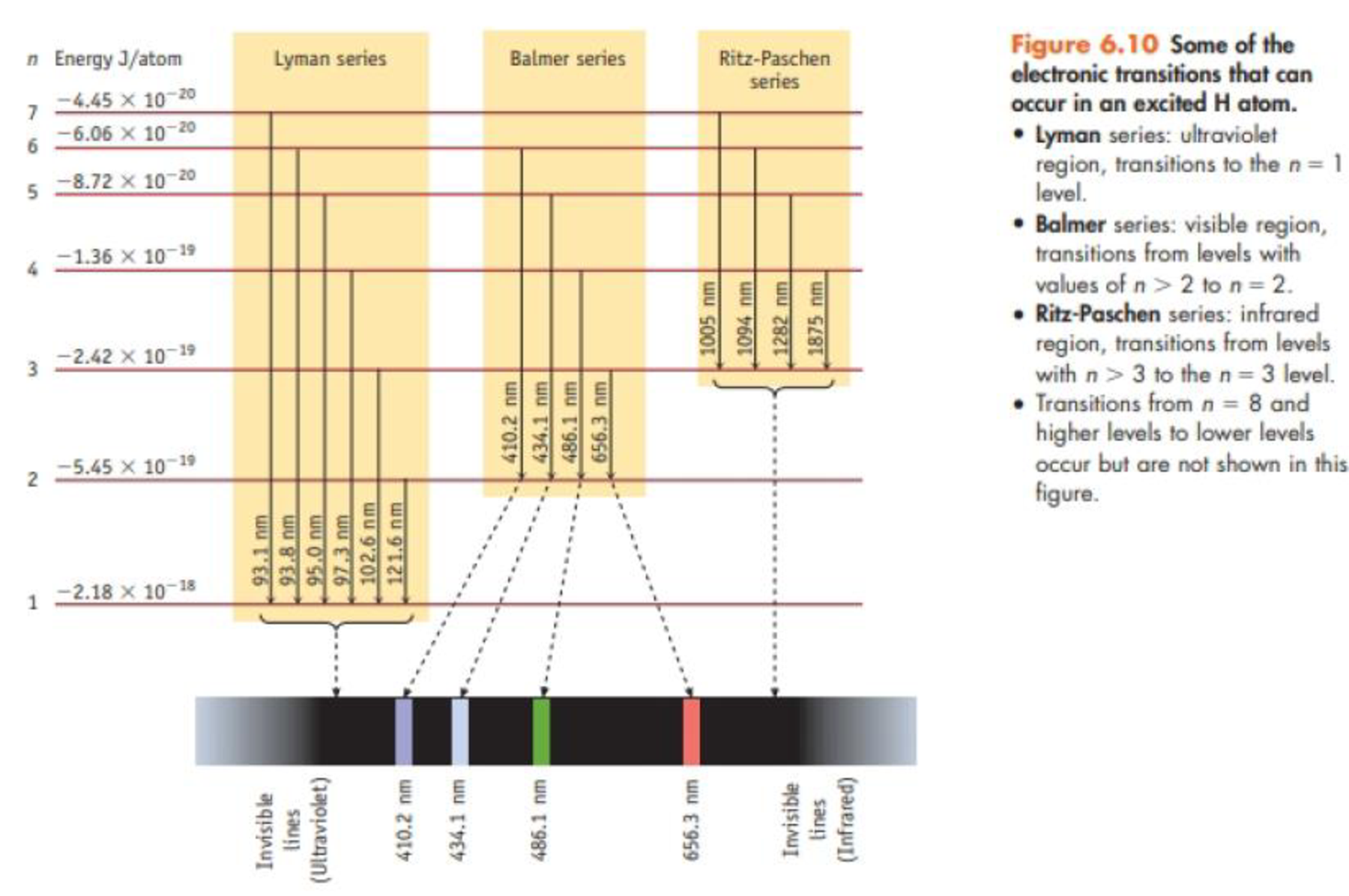
Consider only transitions involving the n = 1 through n = 5 energy levels for the H atom (see Figures 6.7 and 6.10).
- (a) How many emission lines are possible, considering only the five quantum levels?
- (b) Photons of the highest frequency are emitted in a transition from the level with n = ______ to a level with n = ______.
- (c) The emission line having the longest wavelength corresponds to a transition from the level with n = ______ to the level with n = _________.
Expert Solution & Answer
Trending nowThis is a popular solution!

Chapter 6 Solutions
Chemistry & Chemical Reactivity
Ch. 6.1 - (a) Which color in the visible spectrum has the...Ch. 6.1 - 1. Which of the following types of electromagnetic...Ch. 6.1 - What is the wavelength of an FM radio signal that...Ch. 6.2 - Calculate the energy per mole of photons for the...Ch. 6.2 - Prob. 1RCCh. 6.2 - Prob. 2RCCh. 6.3 - Prob. 1CYUCh. 6.3 - The Lyman series of spectral lines for the H atom,...Ch. 6.3 - 1. Based on Bohr's theory, which of the following...Ch. 6.3 - Based on Bohr's theory, which of the following...
Ch. 6.4 - Calculate the wavelength associated with a neutron...Ch. 6.4 - 1. The wavelength associated with an electron...Ch. 6.5 - 1. What label is given to an orbital with quantum...Ch. 6.5 - 2. How many orbitals are in the n = 4...Ch. 6.5 - Prob. 3RCCh. 6.6 - 1. Which of the following is not a correct...Ch. 6.6 - Which of the following sets of quantum numbers...Ch. 6.6 - How many nodal planes exist for a 5d orbital? (a)...Ch. 6.7 - Which of the following is a valid set of quantum...Ch. 6 - Answer the following questions based on Figure...Ch. 6 - Consider the colors of the visible spectrum. (a)...Ch. 6 - Traffic signals are often now made of LEDs...Ch. 6 - Suppose you are standing 225 m from a radio...Ch. 6 - Green light has a wavelength of 5.0 102 nm. What...Ch. 6 - Violet light has wavelength of about 410 nm. What...Ch. 6 - The most prominent line in the emission spectrum...Ch. 6 - The most prominent line in the emission spectrum...Ch. 6 - Place the following types of radiation in order of...Ch. 6 - Place the following types of radiation in order of...Ch. 6 - An energy of 3.3 1019 J/atom is required to cause...Ch. 6 - You are an engineer designing a switch that works...Ch. 6 - The most prominent line in the spectrum of mercury...Ch. 6 - The most prominent line in the spectrum of neon is...Ch. 6 - A line in the Balmer series of emission lines of...Ch. 6 - What are the wavelength and frequency of the...Ch. 6 - Consider only transitions involving the n = 1...Ch. 6 - Consider only transitions involving the n = 1...Ch. 6 - The energy emitted when an electron moves from a...Ch. 6 - If energy is absorbed by a hydrogen atom in its...Ch. 6 - Calculate the wavelength and frequency of light...Ch. 6 - Calculate the wavelength and frequency of light...Ch. 6 - An electron moves with a velocity of 2.5 X 108...Ch. 6 - A beam of electrons (m = 9.11 X 1031 kg/electron)...Ch. 6 - Calculate the wavelength, in nanometers,...Ch. 6 - A rifle bullet (mass = 1.50 g) has a velocity of...Ch. 6 - (a) When n = 4, what are the possible values of ?...Ch. 6 - (a) When n = 4, = 2, and m = 1, to what orbital...Ch. 6 - A possible excited state of the H atom has the...Ch. 6 - A possible excited state for the H atom has an...Ch. 6 - How many subshells occur in the electron shell...Ch. 6 - Prob. 32PSCh. 6 - Explain briefly why each of the following is not a...Ch. 6 - Which of the following represent valid sets of...Ch. 6 - What is the maximum number of orbitals that can be...Ch. 6 - What is the maximum number of orbitals that can be...Ch. 6 - Explain briefly why each of the following is not a...Ch. 6 - Explain briefly why each of the following is not a...Ch. 6 - State which of the following orbitals cannot exist...Ch. 6 - State which of the following orbitals cannot exist...Ch. 6 - Write a complete set of quantum numbers (n, , m)...Ch. 6 - Write a complete set of quantum numbers (n, , and...Ch. 6 - A particular orbital has n = 4 and = 2. What must...Ch. 6 - A given orbital has a magnetic quantum number of m...Ch. 6 - Prob. 45PSCh. 6 - Prob. 46PSCh. 6 - Which of the following are applicable when...Ch. 6 - Prob. 48GQCh. 6 - Give the number of nodal surfaces through the...Ch. 6 - What is the maximum number of s orbitals found in...Ch. 6 - Match the values of l shown in the table with...Ch. 6 - Sketch a picture of the 90% boundary surface of an...Ch. 6 - Complete the following table.Ch. 6 - Excited H atoms have many emission lines. One...Ch. 6 - An advertising sign gives off red light and green...Ch. 6 - Radiation in the ultraviolet region of the...Ch. 6 - A cell phone sends signals at about 850 MHz (where...Ch. 6 - Assume your eyes receive a signal consisting of...Ch. 6 - If sufficient energy is absorbed by an atom, an...Ch. 6 - Suppose hydrogen atoms absorb energy so that...Ch. 6 - Rank the following orbitals in the H atom in order...Ch. 6 - How many orbitals correspond to each of the...Ch. 6 - Cobalt-60 is a radioactive isotope used in...Ch. 6 - Exposure to high doses of microwaves can cause...Ch. 6 - When the Sojourner spacecraft landed on Mars in...Ch. 6 - The most prominent line in the emission spectrum...Ch. 6 - Answer the following questions as a summary quiz...Ch. 6 - Answer the following questions as a summary quiz...Ch. 6 - For an electron in a hydrogen atom, calculate the...Ch. 6 - A solution of KMnO4 absorbs light at 540 nm (page...Ch. 6 - Prob. 71ILCh. 6 - The spectrum shown here is for aspirin. The...Ch. 6 - The infrared spectrum for methanol. CH3OH, is...Ch. 6 - Bohr pictured the electrons of the atom as being...Ch. 6 - Light is given off by a sodium- or...Ch. 6 - Prob. 76SCQCh. 6 - What does wave-particle duality mean? What are its...Ch. 6 - Prob. 79SCQCh. 6 - Suppose you live in a different universe where a...Ch. 6 - A photon with a wavelength of 93.8 nm strikes a...Ch. 6 - Explain why you could or could not measure the...Ch. 6 - Prob. 83SCQ
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A particular microwave oven delivers 750 watts. (A watt is a unit of power, which is the joules of energy delivered, or used, per second.) If the oven uses microwave radiation of wavelength 12.6 cm, how many photons of this radiation are required to heat 1.00 g of water 1.00C, assuming that all of the photons are absorbed?arrow_forwardThe energy emitted when an electron moves from a higher energy state to a lower energy state in any atom can be observed as electromagnetic radiation. (a) Which involves the emission of less energy in the H atom, an electron moving from n = 4 to n = 2 or an electron moving from n = 3 to n = 2? (b) Which involves the emission of more energy in the H atom, an electron moving from n = 4 to n = 1 or an electron moving from n = 5 to n = 2? Explain fully.arrow_forwardLight with a wavelength of 405 nm fell on a strontium surface, and electrons were ejected. If the speed of an ejected electron is 3.36 105 m/s, what energy was expended in removing the electron from the metal? Express the answer in joules (per electron) and in kilojoules per mole (of electrons).arrow_forward
- Light with a wavelength of 425 nm fell on a potassium surface, and electrons were ejected at a speed of 4.88 105 m/s. What energy was expended in removing an electron from the metal? Express the answer in joules (per electron) and in kilojoules per mole (of electrons).arrow_forward6.17 The laser in most supermarket barcode scanners operates at a wavelength of 632.8 nm. What is the energy of a single photon emitted by such a laser? What is the energy of one mole of these photons?arrow_forward6.29 A mercury atom emits light at many wavelengths, two of which are at 435.8 and 546.1 nm. Both of these transitions are to the same final state. (a) What is the energy difference between the two states for each transition? (b) lf a transition between the two higher energy states could be observed, what would be the frequency of the light?arrow_forward
- A hydrogen atom in the ground stale absorbs a photon whose wavelength is 95.0 nm. The resulting excited atom then emits a photon of 1282 nm. What are the regions of the electromagnetic spectrum for the radiations involved in these transitions? What is the principal quantum number of the final state resulting from the emission from the excited atom?arrow_forward6.85 The visible lines in the hydrogen atom emission spectrum arise from transitions with a final state with n = 2. In what spectral region should we expect to find transitions that have a final state of n = 1 ? Explain your reasoning using an energy level diagram similar to the one in Problem 6.26.arrow_forward6.96 When a helium atom absorbs light at 58.44 nm, an electron is promoted from the 1s orbital to a 2p orbital. Given that the ionization energy of (ground state) helium is 2372 kJ/ mol, find the longest wavelength of light that could eject an electron from the excited state helium atom.arrow_forward
- Three emission lines involving three energy levels in an atom occur at wavelengths x, 1.5x, and 3.0x nanometers. Which wavelength corresponds to the transition from the highest to the lowest of the three energy levels?arrow_forwardWarm objects emit electromagnetic radiation in the infrared region. Heat lamps employ this principle to generate infrared radiation. Water absorbs infrared radiation with wavelengths near 2.80 m. Suppose this radiation is absorbed by the water and converted to heat. A 1.00-L sample of water absorbs infrared radiation, and its temperature increases from 20.0C to 30.0C. How many photons of this radiation are used to heat the water?arrow_forward6.14 For photon with the following energies, calculate the wavelength and identify the region of the spectrum they are from. (a) 3.51020 J, (b) 8.71026 J, (c) 7.11017 J, (d) 5.51027 Jarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry; Author: Crash Chemistry Academy;https://www.youtube.com/watch?v=apuWi_Fbtys;License: Standard YouTube License, CC-BY