
ORGANIC CHEMISTRY
5th Edition
ISBN: 9781259977596
Author: SMITH
Publisher: MCG
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 6, Problem 6.12P
The equilibrium constant for the conversion of the axial to the equatorial conformation of methoxycyclohexane is
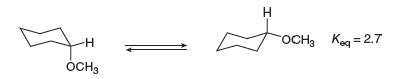
a. Given these data, which conformation is present in the larger amount at equilibrium?
b. Is
c. From the values in Table
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Given the following data:
4C(s) + 4H2(g) + O2(g) → CH3CH2OCOCH3(l)
ΔH°=-480.0 kJ
CH3CH2OH(l) + O2(g) → CH3COOH(l) + H2O(l)
ΔH°=-492.0 kJ
2C(s) + 3H2(g) + 1/2O2(g) → CH3CH2OH(l)
ΔH°=-278.0 kJ
H2(g) + 1/2O2(g) → H2O(l)
ΔH°=-286.0 kJ
calculate ΔH° for the reaction:CH3COOH(l) + CH3CH2OH(l) → CH3CH2OCOCH3(l) + H2O(l)
Given the following data:
2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(l) ΔG0= -6399 kJ
C(s) + O2(g) → CO2(g) ΔG0= -394 kJ
H2(g) + ½O2(g) → H2O(l) ΔG0= -237 kJ
Calculate the ΔG0rxn for the reaction
6 C(s) + 3 H2(g) → C6H6(l)
5. Calculate AH xn for the combustion of butanol:
CH3CH2CH2CH2OH (I) + 6 O2 (g)→4 CO2 (g) +5 H2O (g)
Compound
AH"r (kJ/mol)
C4H9OH (I)
-332.8
CO2 (g)
-393.5
H20 (g)
-241.8
Chapter 6 Solutions
ORGANIC CHEMISTRY
Ch. 6 - Problem 6.1 Classify each transformation as...Ch. 6 - Prob. 6.2PCh. 6 - Problem 6.3 By taking into account...Ch. 6 - Problem 6.4 Use curved arrows to show the movement...Ch. 6 - Problem 6.5 Follow the curved arrows and draw the...Ch. 6 - Prob. 6.6PCh. 6 - Problem 6.7 Use the values in Table 6.2 to...Ch. 6 - Prob. 6.8PCh. 6 - aWhich Keq corresponds to a negative value of G,...Ch. 6 - Given each of the following values, is the...
Ch. 6 - Given each of the following values, is the...Ch. 6 - The equilibrium constant for the conversion of the...Ch. 6 - Prob. 6.13PCh. 6 - For a reaction with H=40kJ/mol, decide which of...Ch. 6 - For a reaction with H=20kJ/mol, decide which of...Ch. 6 - Draw an energy diagram for a reaction in which the...Ch. 6 - Prob. 6.17PCh. 6 - Prob. 6.18PCh. 6 - Problem 6.19 Consider the following energy...Ch. 6 - Draw an energy diagram for a two-step reaction,...Ch. 6 - Which value if any corresponds to a faster...Ch. 6 - Prob. 6.22PCh. 6 - Problem 6.23 For each rate equation, what effect...Ch. 6 - Prob. 6.24PCh. 6 - Identify the catalyst in each equation. a....Ch. 6 - Draw the products of homolysis or heterolysis of...Ch. 6 - Explain why the bond dissociation energy for bond...Ch. 6 - Classify each transformation as substitution,...Ch. 6 - Prob. 6.29PCh. 6 - 6.30 Draw the products of each reaction by...Ch. 6 - 6.31 (a) Add curved arrows for each step to show...Ch. 6 - Prob. 6.32PCh. 6 - Prob. 6.33PCh. 6 - Prob. 6.34PCh. 6 - Calculate H for each reaction. a HO+CH4CH3+H2O b...Ch. 6 - Homolysis of the indicated CH bond in propene...Ch. 6 - Prob. 6.37PCh. 6 - Prob. 6.38PCh. 6 - 6.39. a. Which value corresponds to a negative...Ch. 6 - Prob. 6.40PCh. 6 - For which of the following reaction is S a...Ch. 6 - Prob. 6.42PCh. 6 - Prob. 6.43PCh. 6 - 6.44 Consider the following reaction: .
Use curved...Ch. 6 - Prob. 6.45PCh. 6 - Draw an energy diagram for the Bronsted-Lowry...Ch. 6 - Prob. 6.47PCh. 6 - Indicate which factors affect the rate of a...Ch. 6 - Prob. 6.49PCh. 6 - 6.50 The conversion of acetyl chloride to methyl...Ch. 6 - Prob. 6.51PCh. 6 - Prob. 6.52PCh. 6 - The conversion of (CH3)3Cl to (CH3)2C=CH2 can...Ch. 6 - 6.54 Explain why is more acidic than , even...Ch. 6 - Prob. 6.55PCh. 6 - Prob. 6.56PCh. 6 - Prob. 6.57PCh. 6 - Although Keq of equation 1 in problem 6.57 does...Ch. 6 - Prob. 6.59P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- For the complete combustion of 1.000 mole of propane gas at 298 K and 1 atm pressure, ∆H° = -2220 kJ/mol. What will be the heat released when 4.13 g of propane is combusted under these conditions? The chemical formula for propane is C3H8.arrow_forwardConsider these hydrocarbons: Butane, Hexane, Methane, Propane First look up the formulas, and write them down. Then put them in order from least entropy to most entropy. (Assume the temperature is hot enough that all are in gaseous form.)arrow_forwardced Use the References to access important values if needed for this question. Complete the following reaction by drawing structural formulas for the organic product(s). CH3 0 CH3CHCH₂COCH3 ● • You do not have to consider stereochemistry. . If there are two different organic products, draw both. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. [... + H₂O CH4 H+ ChemDoodle Previousarrow_forward
- Calculate ΔG° for the reaction of benzene with hydrogen gas to give cyclohexane using the following data: ?6?6 + 3H2 → ?6?12 ΔG∘f (benzene) = 124.5 kJ/mol ΔG∘f (cyclohexane) = 217.3 kJ/mol. Is the reaction spontaneous as written?arrow_forwardTwo 3-carbon molecules are shown here. Both molecules can be completely broken down to CO2 in a process that releases free energy. Which molecule has more free energy to release(left or right)? Explain why.arrow_forwardAcetylene burns in air according to the following equation. C2H2(g) + 5/2 02(g) → 2 CO2(g) + H20(g) AH°, rxn = -1255.8 k) Given that AHO of CO2(g) = -393.5 kJ/mol and AH°; of H20(g) = -241.8 kJ/mol, what is AHO, of C2H2(g)? 4.0 Your response differs from the correct answer by more than 10%. Double check your calculations. kJ/mol Submit Answerarrow_forward
- In glycolysis, the reaction of glucose (Glu) to form glucose-6-phosphate (G6P) requires ATP to be present as described by the following equation: Glu+ ATP→G6) + ADP AG° = - 17KJ In this process, ATP becomes ADP summarized by the following equation: ATP→ ADP A G° =- 30 kJ What is the standard free energy change for the following reaction: Glu →G6P AG° =? A 17 k) B) -13 k) c) 13 kJ -17 kJarrow_forwardConsider the following reaction: 2CH₂OH(g)→2CH₂(g) + O₂(g), AH- +252.8 kJarrow_forwardGiven that the reaction of 4 NH3 (g) + 5 O2 (g) → 4 NO (g) + 3 H20 (g) ΔΗ - - 906 kJ What would AH for NO (g) + ? H20 (g) → NH3 (g) + O2 (g) be? 2. 906 kJ 226.5 kJ -226.5kJ 453 kJarrow_forward
- Assume that the complete combustion of one mole of glucose, a monosaccharide, to carbon dioxide and water liberates 2870 kJ of energy (AG°¹ = −2870 kJ/mol). If the energy generated by the combustion of glucose is entirely converted to the synthesis of a hypothetical compound X, calculate the number of moles of the compound that could theoretically be generated. Use the value AGo' compound X = -54.9 kJ/mol. Round your answer to two significant figures. moles: =arrow_forwardQ7. Some spontaneous reactions take 20 million years for half of the reactants to be converted into products while other reactions take 10 seconds. What is the basis for this difference in rate? A. Reactions take longer the closer their ΔG is to zero. B. The rate is dependent upon the concentration of substrate molecules. C. Reactions where substrate molecules can more readily reach the transition state occur faster. D. The rate of the reaction is dependent upon a supply of energy - thus coupled reactions that utilize ATP are the fastest.arrow_forwardpetrol has a Molar mass of 114 g.mol-1 and a ΔcH0 of -5470 kJ.mol-1. calculate the energy density of petrol in (in kJ.g-1)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY