
Rearrange the Clausius-Clapeyron equation, equation 6.14 in terms of the pressure
Interpretation:
The vapor pressures of
Concept introduction:
The Clausius-Clapeyron equation can be obtained from the rearrangement and integration of Clapeyron equation. The Clausius-Clapeyron equation is generally used for gas-phase equilibria, to predict the equilibrium temperatures and pressures and also for the determination of enthalpy for phase transition.
Answer to Problem 6.70E
The plot between vapor pressure and temperature for
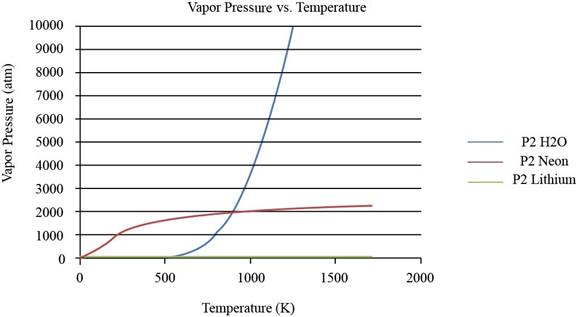
Figure 1
From the plot, the common observation is that for the given substances the increase in vapor pressure is slow until the normal boiling point.
Explanation of Solution
The Clausius-Clapeyron equation 6.14 is,
Rearrange the given equation for the partial pressure
Given boiling point of
Calculation of partial pressure
Calculation of partial pressure
Calculation of partial pressure
The plot between vapor pressure and temperature for
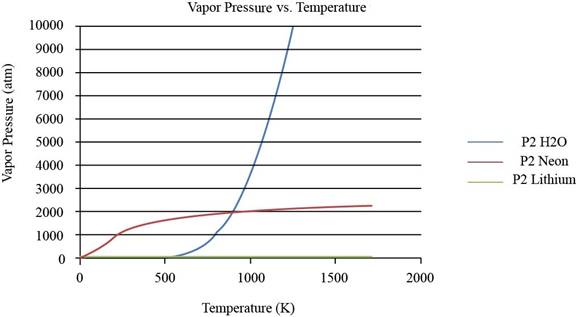
Figure 1
To observe the change in the plot of lithium, the values of vapor pressure is considered till
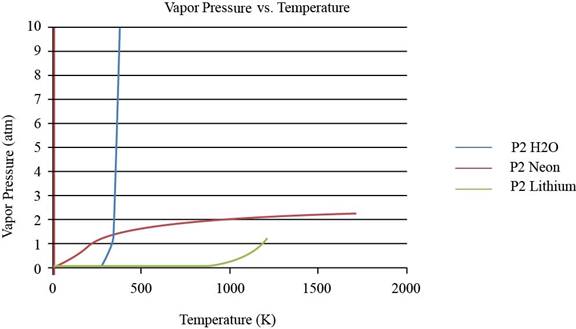
Figure 2
From the plot, the common observation is that for the given substances the increase in vapor pressure is slow until the normal boiling point. After the normal boiling point, the increase in vapor pressure is exponential. As the temperature increases from lower normal boiling point to higher values, the exponent value changes from negative to positive.
From the plot, the common observation is that for the given substances, the increase in vapor pressure is slow until the normal boiling point.
Want to see more full solutions like this?
Chapter 6 Solutions
Physical Chemistry
- The normal boiling point of SO2 is 263.1 K and that of NH3 is 239.7 K. At −40 °C, would you predict that ammonia has a vapor pressure greater than, less than, or equal to that of sulfur dioxide? Explain.arrow_forwardDescribe, in general, the structures of ionic solids. Compare and contrast the structure of sodium chloride and zinc sulphide. How many tetrahedral holes and octahedral holes are there per closest packed anion? In zinc sulphide, why are only one-half of the tetrahedral holes filled with cations?arrow_forwardArrange the following substances in order of increasing strength of crystal forces: CO2, KCl, H2O, N2, CaO.arrow_forward
- If you place 30.0 L of hexane (C6H14) in a sealed room that is 3.75 m long, 2 m wide, and 3 m high, will all the hexane evaporate? If some liquid remains, how much will there be? The vapor pressure of hexane is 152 torr at 25 °C, and the density of the liquid at this temperature is 0.655 g/mL. Treat the room dimensions as exact numbers.arrow_forward3.6 g of methane is collected over water at STP for a total of 4.27 L at 25 degrees Celsius. What is the vapor pressure of water at this temperature?arrow_forwardWhich of the following forces can be used to explain why F2 and Cl₂ are gases, whereas Br2 is a liquid and 12 is a solid at regular condition (25 °C & 1 atm)?arrow_forward
- 1. A 1.70 m3 container holds a sample of O2 gas. At 294 K the pressure of the container is 1.08 x 105 Pa. How many moles of O2 are in the container? (Enter your answer as a number only) 2. Classify each of the following substances, in the solid state, as molecular, ionic, covalent network or metallic. Substance Appearance Melting Pt. (°C) Boiling Pt. (°C) Conducts as solid Conducts as liquid A grey 795 3468 yes yes B colorless crystals 87 220 no no C colorless crystals 1713 2230 no no D yellowish hexagonal crystal 740 1100 no yes A B D C 1. metallic 2. molecular 3. covalent network 4. ionic what is A B C Darrow_forwardCalcium forms a face-centered cubic cell with a density of 1.54 g/ml, what would its radius be in picometers?arrow_forwardUsing the graph of ln P vs 1/T (in Kelvin) for water vapor pressure, what is the vapor pressure of water at 15.0℃? Now take the exponential of ln P to get the vapor pressure of water at 15.0℃ in units of mmHg. a. 758.5 b. 745.7 c. 724.7 d. none of these e. 735.0 f. 735.4 g. 748.2 h. 760.0 i. 12.84087 j. 23.53877arrow_forward
- Why does water form a concave lens in a test tube, whereas mercury forms a convex lens.arrow_forwardWhat is the simplest formula of a solid containing A, B, C atoms in a cubic lattice in which A atoms occupy the corners, the B atom is the body-centered position, and the C atoms the faces of the unit cell?arrow_forwardA 6.30 g sample of solid Na3PO4⋅7H2O was heated such that the water turned to steam and was driven off. Assuming ideal behavior, what volume would that steam occupy at 1.00 atm and 100.0 °C?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





