
Concept explainers
PRACTICE PROBLEM 6.6
(a) Illustrate how this is true by assigning R, S configurations to the 2-chlorobutane enantiomers based on the following data. [The configuration of (–)-2-butanol is given in Section 5.8C.]
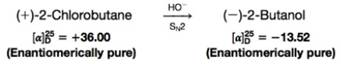
(b) When enantiomerically pure (+)-2-chlorobutanc is allowed to react with potassium iodide in acetone in an
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
Chemistry & Chemical Reactivity
Elementary Principles of Chemical Processes, Binder Ready Version
Chemistry: A Molecular Approach
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
- • PRACTICE PROBLEM 8.12 Treating a hindered alkene such as 2-methyl-2-butene with BH3:THE leads to the for- mation of a dialkylborane instead of a trialkylborane. When 2 mol of 2-methyl-2-butene is added to 1 mol of BH3, the product formed is bis(3-methyl-2-butyl)borane, nicknamed in reagent "disiamylborane." Write its structure. Bis(3-methyl-2-butyl)borane is a useful certain syntheses that require a sterically hindered borane. (The name “disiamyl" comes from "disecondary-iso-amyl," a completely unsystematic and unacceptable name. The name "amyl" is an old common name for a five-carbon alkyl group.) donbyarrow_forwardName each alkene and specify its configuration by the E,Z system. (Be sure to indicate double bond stereochemistry using (E)/(Z) notation. It is not necessary to use italics in writing compound names.) (a) (b) (C) کو C1- Br لولهarrow_forward6.26 There is an overall 29-fold difference in reactivity of 1-chlorohexane, 2-chlorohexane, and 3-chlorohexane toward potassium iodide in acetone. (a) Which one is the most reactive? Why? (b) Two of the isomers differ by only a factor of 2 in reactivity. Which two are these? Which one is the more reactive? Why?arrow_forward
- • PRACTICE PROBLEM 8.13 Specify the appropriate alkene and reagents for synthesis of each of the following alcohols by hydroboration–oxidation. (a) (c) OH (e) CH3 OH AH OH no mobe OH ( (b) (d) (f) OH HT H3 D OH OH HO OHarrow_forward4.2 10 Give the reaction mechanism showing all possible products for the following reactions: (a) Reaction of 1-propene with hydrogen bromide, HBr (g) (b) Reaction of ethene with bromine, Br₂ (1)arrow_forwardO PRACTICE PROBLEM 8.14 Starting with any needed alkene (or cycloalkene) and assuming you have deuterioace- tic acid (CH3CO,D) available, outline syntheses of the following deuterium-labeled compounds.s el en olad lo nohibbs ad CH3 (a) (CH3)2CHCH2CH,D (b) (CH3),CHCHDCH3 (c) (+ enantiomer) (d) Assuming you also have available BD3:THF and CH3CO2T, can you suggest a synthesis of the following? hab erl (+ enantiomer)he imo (nwond-ben) CH3 H. (asoholea)arrow_forward
- 7.44 Give the structures of two different alkyl bromides both of which yield the indicated alkene as the exclusive product of E2 elimination. (а) СH;CH—CH, (b) (CH3)2С—СH2 (c) BRCH=CB12 CH3 (d) CH3arrow_forward6.20 Account for the regioselectivity and stereoselectivity observed when 1-methylcyclopentene is treated with each reagent. (a) BH, (b) Br₂ in H₂O (c) Hg(OAc), in H₂O 6.21 Draw a structural formula for an alkene with the indicated molecular formula that ion product (more than one alkene may give thearrow_forward(a) H. H. 6.44 Which of the following reactions will yield a racemic mixture of products? HCI HBr (a) (c) H2 H2 (b) (d) Pt Ptarrow_forward
- 6.51b Explain the observed stereochemistry. When the second intermediate is redrawn from the following perspective, it becomes clear that O the electrons in the carbon-carbon-bond come from above the carbon-axygen x bond, allowing for the chirality center to be generated with the stereochemistry shown. the electrons in the carbon-carbon-bond come from below the carbon-oxygen x bond, allowing for the chirality center to be generated with the stereochemistry shown. the electrons in the carbon-exygen-bond come from below the carbon-cartion a bond, allowing for the chirality center to be generated with the stereochemistry shown. the electrons in the carbon-exygen x-band come from above the carbon-carbon bond, allowing for the chirality center to be generated with the stereochemistry shown. OOarrow_forwardConsider the reaction between (1S,3S)‑1‑chloro‑3‑methylcyclopentane and methanethiol in the presence of sodium hydroxide. (a) Draw the organic product and clearly indicate stereochemistry by showing the hydrogen on the chirality centers and using wedge and dash bonds. (b) Then analyze the stereochemistry of the product. racemic chiral achiral (1R, 3S) (1R, 3R) (1S, 3S)arrow_forward7.1 Provide the line structures of the products formed in the following reactions. One block may represent more than one product. (a) HBr Diethyl ether (b) H,0, H*arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





