
Concept explainers
(a)
Interpretation:
The graph of the given data to form an oxygen-binding curve is to be plotted. The oxygen partial pressure at which the given hemoglobin is half-saturated is to be stated. Whether oxygen binding seems to be cooperative or not on the basis of the curve is to be stated.
Concept introduction:
Proteins are the
Answer to Problem 16P
The plot of the given data to form an oxygen-binding curve is shown below.
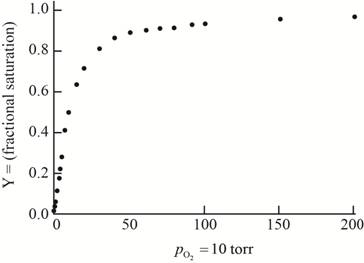
The oxygen partial pressure at which the given hemoglobin is half-saturated is
Explanation of Solution
On the basis of the given data for oxygen-binding for lamprey hemoglobin, the graph of fractional saturation versus partial pressure of oxygen is plotted as follows:
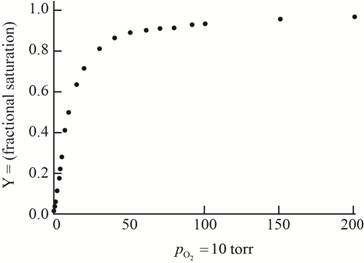
At the oxygen partial pressure,
Thus, this plot between partial pressure of oxygen
(b)
Interpretation:
A hill plot by using the given data is to be stated. Whether the hill plot shows any evidence for cooperativity or not is to be stated. The information about the hill coefficient is to be explained.
Concept introduction:
Proteins are the biomolecules which are composed of the long chain of amino acid residues. The protein which contains oxygen and is present in the red blood cells in the body is known as hemoglobin. It contains iron as well.
The hill equation indicates the two close equations which show the binding of a ligand with the macromolecule.
Answer to Problem 16P
A hill plot by using the given data is shown as,
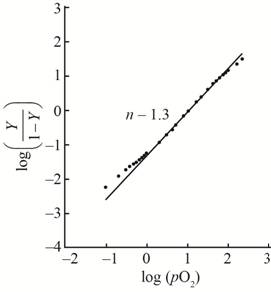
This plot show little bit cooperativity in the center of the curve and its hill coefficient is
Explanation of Solution
The equation that is used to show the hill equation is given as,
Here,
The values of
Similarly, for
The value of
Similarly, for
With the help of the given data for oxygen-binding for lamprey hemoglobin, the plot between
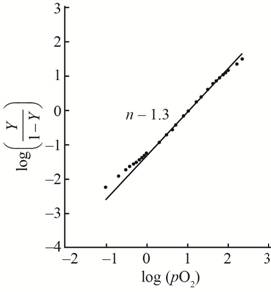
The value of slope
The hill coefficient for the graph is
(c)
Interpretation:
A model that is used to explain the observed cooperativity in oxygen binding by lamprey hemoglobin is to be stated.
Concept introduction:
Proteins are the biomolecules which are composed of the long chain of amino acid residues. The protein which contains oxygen and is present in the red blood cells in the body is known as hemoglobin. It contains iron as well.
Answer to Problem 16P
A model that is used to explain the observed cooperativity in oxygen binding by lamprey hemoglobin suggests that binding of oxygen with any monomer is easier as compared to binding of first oxygen with deoxygenated dimer.
Explanation of Solution
It is given that lamprey hemoglobin produces oligomers in the deoxygenated state. These oligomers were primarily dimmers in the deoxygenated state. These dimers are having low affinity for oxygen as compared to monomers. The cooperativity in oxygen binding is shown only if the dissociation of dimmers into two monomers occurs after the binding of first oxygen atom with a dimer. In this model, the binding of oxygen with every monomer is very easy as compared to the binding between first oxygen and the deoxy dimer.
Want to see more full solutions like this?
- Catabolism - draw the complete citric acid cylcle for myristatearrow_forwardOxygen-hemoglobin curve dissociation curve: Which various factors can determine the amount of saturation? why is this curve so steep? how ph and temperature affect it? how does the binding of oxygen molecules in bohr and haldane effect affect the 3d shape of hemoglobin and how does that have to do with the curve?arrow_forwardMolecular detail of spike Y453Farrow_forward
- Myoglobin’s oxygen-binding curve exhibits a shape best described as— serpentine. hyperbolic. straight. sigmoidal. bell.arrow_forward(No more than one page). Explain why quaternary structure is necessary for cooperativity and allosteric regulation. Use hemoglobin as an example. Include the various allosteric effectors that influence oxygen binding.arrow_forwardA variant of hemoglobin (Boston variant; mutation His E7(58)α → Tyr) promotes methemoglobin formation involving the α (alpha) subunits. What is the maximum value of the Hill constant (n) that you could measure for the Boston variant of hemoglobin? log (YO2 / 1 - YO2 ) = log pO2 - logP50 Please break down each step of the Hill equation and explain why the result for n is valid from a logical standpoint.arrow_forward
- Comparison of Fetal and Maternal Hemoglobins.Studies of oxygen transport in pregnant mammals show that the O2-saturation curves of fetal and maternal blood are markedly different when measured under the same conditions. Fetal erythrocytes contain a structural variant of hemoglobin, HbF, consisting oftwo a and two g subunits (α2γ2), whereas maternal erythrocytes contain HbA (α2β2). a)Which hemoglobin has a higher affinity for oxygen under physiological conditions, HbA or HbF? Explain. b)What is the physiological significance of the different O2 affinities? c)When all the BPG is carefully removed from samples of HbA and HbF, the measured O2-saturation curves (and consequently the O2affinities) are displaced to the left. However, HbA now has a greater affinity for oxygen than does HbF. When BPG is reintroduced, the O2-saturation curves return to normal, as shown in the graph. What is the effect of BPG on the O2affinity of hemoglobin? How can the above information be used…arrow_forwardBohr for me, not for thee. Does myoglobin exhibit a Bohr effect? Why or why not?arrow_forwardQuestion:- Explain why it makes sense for the PDH complex in liver to be active when dephosphorylated.arrow_forward
- "Hemoglobin can be in either an oxy- or deoxy- state at a given pH. Given the appropriate pKa values, calculate fraction protonated at a given pH" How would I do this?arrow_forwardIDENTIFICATION. One of the B-complex vitamins which is the precursor of Nicotinamide in the coenzyme NAD+. Carrier of the activated amino acids to the ribosomes for incorporation into the growing peptide chain. Molecule that attaches to myosin and actin during muscle contraction.arrow_forward30. The cooperative binding behavior of hemoglobin for oxygen is best explained by... Group of answer choices The movement of the proximal histidine upon O2 binding causes a structural change at the binding interface between subunits The oxidation of Fe2+ to Fe3+ and formation of the superoxide ion causes distortion of the protoporphyrin ring, altering binding interface between subunits The tetrameric nature of hemoglobin's quaternary structure directly confers cooperative binding activity The movement of the distal histidine upon O2 binding causes a structural change at the binding interface between subunits The binding of O2 causes a pH shift that changes the protonation state of amino acids located at the interface between subunits The solubility of hemoglobin in aqueous solution and its insolubility in nonpolar environmentsarrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
