
Concept explainers
(a)
Interpretation:
Synthesis method of the given compound has to be given from the compound which have the same number of carbon atoms.
Concept Introduction:
Addition of water to
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into
The formed enol and ketone are called keto-enol tautomers.
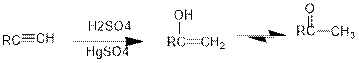
Tautomerization: It is the process of inter conversion of tautomers and therefore the process can also be called as keto-enol tautomerization.
Hydroboration reaction:
It is the reaction in which an internal alkyne is converted into ketone. The reagents used for the hydroboration oxidation reaction is either
(b)
Interpretation:
Synthesis method of the given compound has to be given from the compound which have the same number of carbon atoms.
Concept Introduction:
Addition of water to alkynes in presence of acid:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
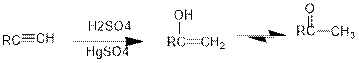
Tautomerization: It is the process of inter conversion of tautomers and therefore the process can also be called as keto-enol tautomerization.
Hydroboration reaction:
It is the reaction in which an internal alkyne is converted into ketone. The reagents used for the hydroboration oxidation reaction is either
(c)
Interpretation:
Synthesis method of the given compound has to be given from the compound which have the same number of carbon atoms.
Concept Introduction:
Addition of water to alkynes in presence of acid:
Under acidic conditions, alkyne reacts with water to produce an enol which then immediately converts into ketone. For terminal alkynes, there is a need of catalyst which is mercury.
The formed enol and ketone are called keto-enol tautomers.
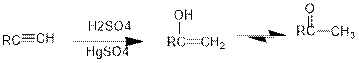
Tautomerization: It is the process of inter conversion of tautomers and therefore the process can also be called as keto-enol tautomerization.
Hydroboration reaction:
It is the reaction in which an internal alkyne is converted into ketone. The reagents used for the hydroboration oxidation reaction is either
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
ETEXT+MASTERINGCHEMISTRY STANDALONE AC
- If one hydrogen in a hydrocarbon is replaced by a halogen atom, the number of isomers that exist for the substituted compound depends on the number of types of hydrogen in the original hydrocarbon. Thus there is only one form of chloroethane (all hydrogens in ethane are equivalent), but there are two isomers of propane that arise from the substitution of a methyl hydrogen or a methylene hydrogen. How many isomers can be obtained when one hydrogen in each of the compounds named below is replaced by a chlorine atom? a. n-pentane b. 2-methylbutane c. 2,4-dimethylpentane d. methylcyclobutanearrow_forwardDraw the structures for two examples of unsaturated hydrocarbons. What structural feature makes a hydrocarbon unsaturated?arrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





